
Aqueous solution chemistry
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 488
الجزء والصفحة:
2th ed p 488
 11-3-2017
11-3-2017
 2138
2138
Aqueous solution chemistry
In this section, we are mainly concerned with redox processes in aqueous solution for a list of relevant topics already covered in the book. Values of Eo for halfreactions 1.1 can be measured directly for X = Cl, Br and I (Table 1.1) and their magnitudes are determined by the X_X bond energies the electron affinities of the halogen atoms (Table 1.1) and the standard Gibbs energies of hydration of the halide ions (Table 1.1).
Table 1.1 Some physical properties of fluorine, chlorine, bromine and iodine.
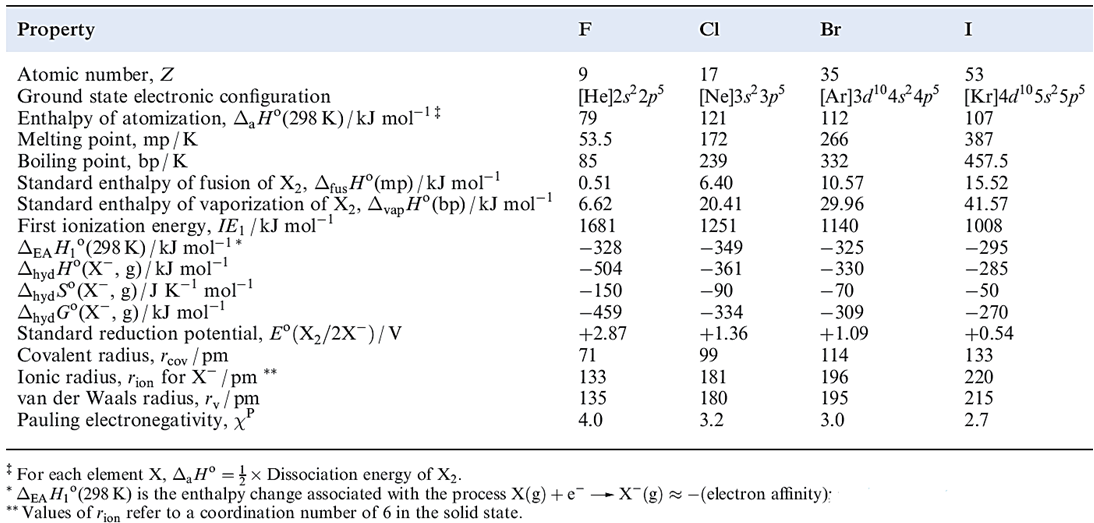
This can be seen from scheme 1.2; for X = Br and I, an additional vaporization stage is needed for the element.
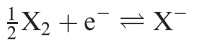 (1.1)
(1.1)
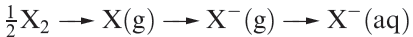 (1.2)
(1.2)
Dichlorine is a more powerful oxidizing agent in aqueous media than Br2 or I2, partly because of a more negative enthalpy of formation of the anion but, more importantly,because the Cl- ion (which is smaller than Br- or I-) interacts more strongly with solvent molecules. (In solid salt formation, the lattice energy factor similarly explains why chloride salts are more exothermic than corresponding bromides or iodides.) Since F2 liberates ozonized O2 from water, the value of Eo for half-reaction 1.1 has no physical reality, but a value of 2.87V can be estimated by comparing the energy changes for each step in scheme 1.2 for X = F and Cl, and hence deriving the difference in Eo for half-equation 1.1 for X = F and Cl. Most of the difference between these Eo values arises from the much more negative value of ΔhydGo of the smaller F- ion (Table 1.1). Diiodine is much more soluble in aqueous iodide solutions than in water. At low concentrations of I2, equation 1.3 describes the system; K can be found be partitioning I2 between the aqueous layer and a solvent immiscible with water (e.g. CCl4).
 (1.3)
(1.3)
Potential diagrams (partly calculated from thermochemical data) for Cl, Br and I are given in Figure 1.1. Because several of the oxoacids are weak, the effects of [H+] on values of some of the reduction potentials are quite complicated. For example, the disproportionation of hypochlorite to chlorate and chloride could be written as equilibrium 1.4 without involving protons.
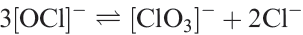 (1.4)
(1.4)
However, the fact that HOCl is a weak acid, while HClO3 and HCl are strong ones means that, in the presence of hydrogen ions, [OCl]- is protonated and this affects the position of equilibrium 1.4: HOCl is more stable with respect to disproportionation than [OCl]- .
On the other hand, the disproportionation of chlorate into perchlorate and chloride is realistically represented by equilibrium 1.5. From the data in Figure 1.1, this reaction is easily shown to be thermodynamically favourable. Nevertheless, the reaction does not occur in aqueous solution owing to some undetermined kinetic factor.
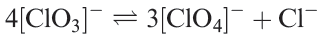 (1.5)
(1.5)
Another example of the limitations of the data in Figure 1.1 is the inference that O2 should oxidize I- and Br- at pH 0. Further, the fact that Cl2 rather than O2 is evolved when hydrochloric acid is electrolysed is a consequence of the high overpotential for O2 evolution at most surfaces. Despite some limitations, Figure 1.1 does provide some useful information: for example, the more powerful oxidizing properties of periodate and perbromate than of perchlorate when these species are being reduced to halate ions, and the more weakly oxidizing powers of iodate and iodine than of the other halates or halogens respectively. The fact that Figure 1.1 refers only to specific conditions is well illustrated by considering the stability of I(I).
Hypoiodous acid is unstable with respect to disproportionation into [IO3]- and I2, and is therefore not formed when [IO3]- acts as an oxidant in aqueous solution.
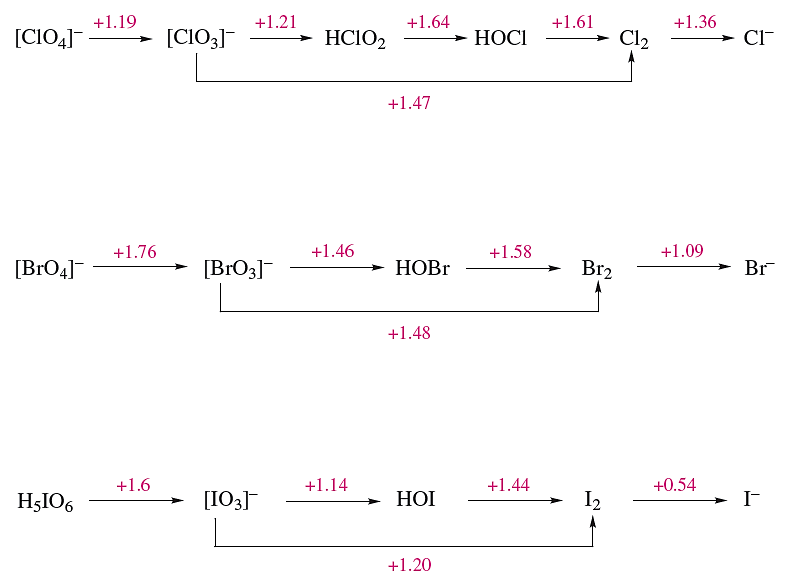
Fig. 1.1 Potential diagrams for chlorine, bromine and iodine at pH = 0.
However, in hydrochloric acid, HOI undergoes reaction 1.6.
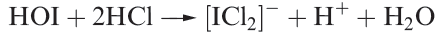 (1.6)
(1.6)
Under these conditions, the potential diagram becomes:
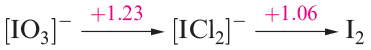
and I(I) is now stable with respect to disproportionation.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


