
Charge transfer complexes
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 477
الجزء والصفحة:
2th ed p 477
 8-3-2017
8-3-2017
 3255
3255
Charge transfer complexes
A charge transfer complex is one in which a donor and acceptor interact weakly together with some transfer of electronic charge, usually facilitated by the acceptor. The observed colours of the halogens arise from an electronic transition from the highest occupied π* MO to the lowest unoccupied σ* MO. The HOMO–LUMO energy gap decreases in the order F2 > Cl2 > Br2 > I2, leading to a progressive shift in the absorption maximum from the near-UV to the red region of the visible spectrum. Dichlorine, dibromine and diiodine dissolve unchanged in many organic solvents (e.g. saturated hydrocarbons, CCl4). However in, for example, ethers, ketones and pyridine, which contain donor atoms, Br2 and I2 (and Cl2 to a smaller extent) form charge transfer complexes with the halogen σ* MO acting as the acceptor orbital. In the extreme, complete transfer of charge could lead to heterolytic bond fission as in the formation of [I)py(2]+ (Figure 16.4 and equation 1.1).
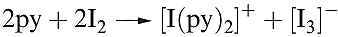 (1.1)
(1.1)
Solutions of I2 in donor solvents, such as pyridine, ethers or ketones, are brown or yellow. Even benzene acts as a donor, forming charge transfer complexes with I2 and Br2; the colours of these solutions are noticeably different from those of I2 or Br2 in cyclohexane (a non-donor). Whereas amines, ketones and similar compounds donate electron density through a σ lone pair, benzene uses its π-electrons; this is apparent in the relative orientations of the donor (benzene) and acceptor (Br2) molecules in Figure 1.1b. That solutions of the charge transfer complexes are coloured means that they absorb in the visible region of the spectrum (≈ 400–750 nm), but the electronic spectrum also contains an intense absorption in the UV region (≈ 230–330 nm) arising from an electronic transition from the solvent _X2 occupied bonding MO to a vacant antibonding MO. This is the socalled charge transfer band. Many charge transfer complexes can be isolated in the solid state and examples are given in Figure 1.1.
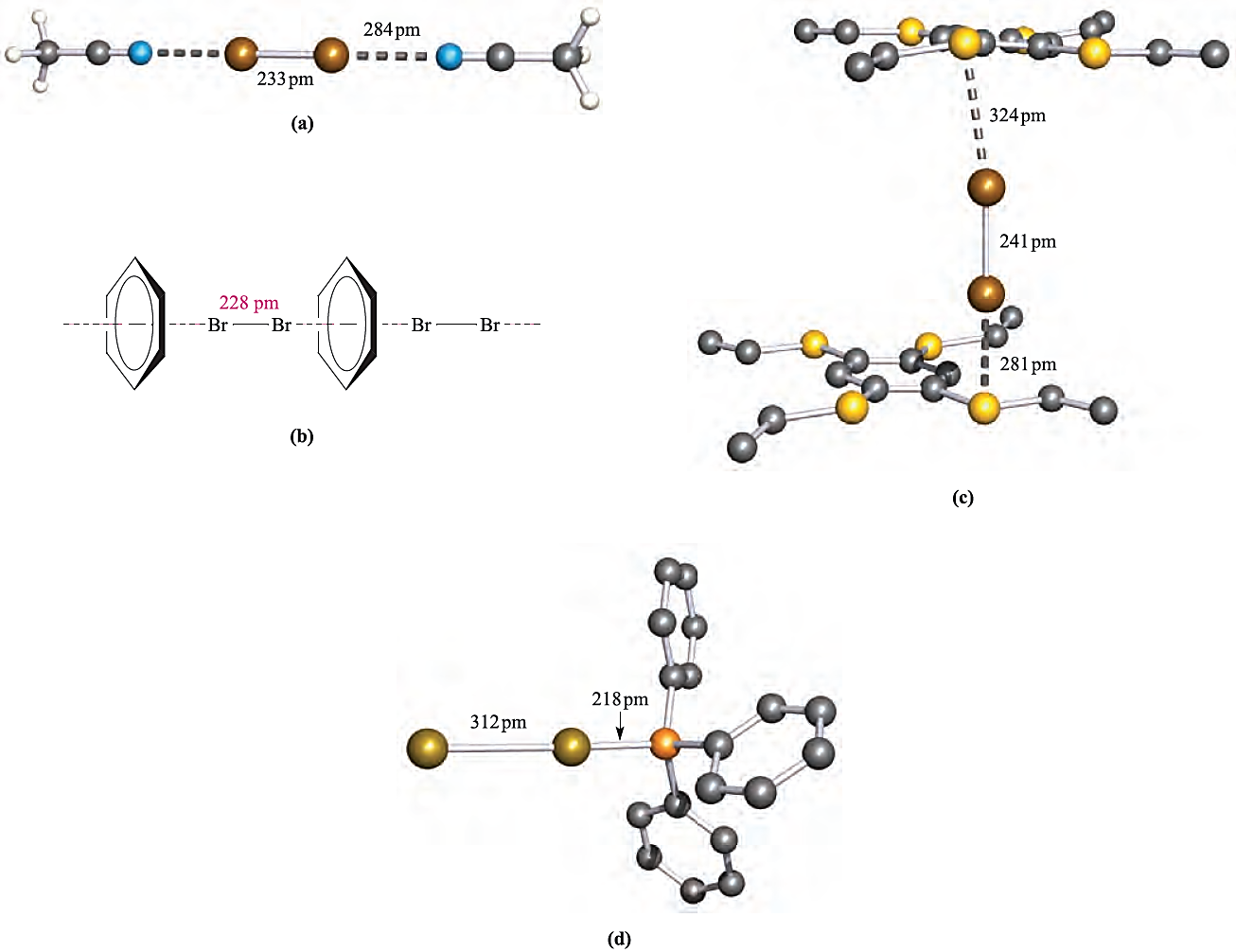
Fig. 1.1 Some examples of charge transfer complexes involving Br2; the crystal structure of each has been determined by X-ray diffraction: (a) 2MeCN.Br2 [K.-M. Marstokk et al. (1968) Acta Crystallogr., Sect. B, vol. 24, p. 713]; (b) schematic representation of the chain structure of C6H6_Br2; (c) 1,2,4,5-(EtS(4C6H2. )Br2(2 in which Br2 molecules are sandwiched between layers of 1,2,4,5-(EtS)4C6H2 molecules; interactions involving only one Br2 molecule are shown and H atoms are omitted [H. Bock et al. (1996) J. Chem. Soc., Chem. Commun., p. 1529]; (d) Ph3P.Br2 [N. Bricklebank et al. (1992) J. Chem. Soc., Chem. Commun., p. 355]. Colour code: Br, brown; C, grey; N, blue; S, yellow; P, orange; H, white.
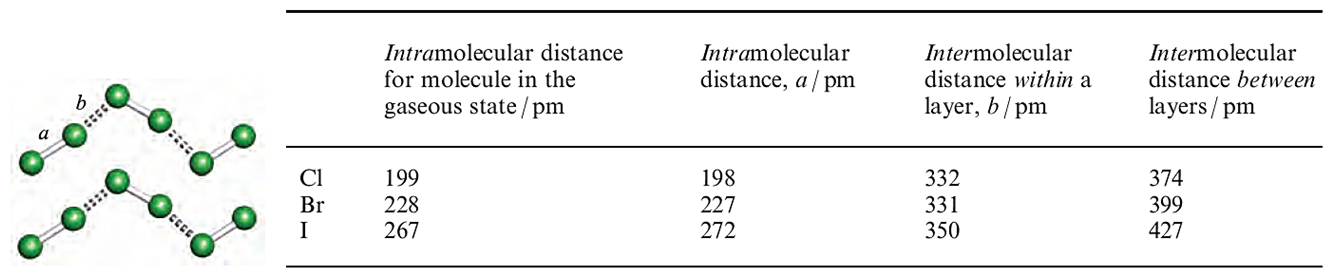
In complexes in which the donor is weak, e.g. C6H6, the X_X bond distance is unchanged (or nearly so) by complex formation. Elongation as in 1,2,4,5-(EtS)4C6H2.(Br2(2 (compare the Br_Br distance in Figure 1.1c with that for free Br2, in Figure in below) is consistent with the involvement of a good donor; it has been estimated from theoretical calculations that _0.25 negative charges are transferred from 1,2,4,5-(EtS)4C6H2 to Br2.
Different degrees of charge transfer are also reflected in the relative magnitudes of ΔrH given in equation 1.2. Further evidence for the weakening of the X_X bond comes from vibrational spectroscopic data, e.g. a shift for ν(X_X( from 215 cm-1 in I2 to 204 cm-1 in C6H6.I2.
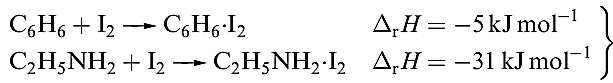 (1.2)
(1.2)
Figure 1.1d shows the solid state structure of Ph3P.Br2; Ph3P.I2 has a similar structure (I_I = 316 pm). In CH2Cl2 solution, Ph3P.Br2 ionizes to give [Ph3PBr]+Br- and, similarly, Ph3PI2 forms [Ph3PI]+I- or, in the presence of excess I2, [Ph3PI]+[I3]-. The formation of complexes of this type is not easy to predict:
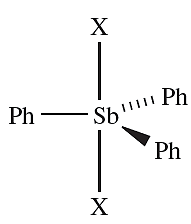
- the reaction of Ph3Sb with Br2 or I2 is an oxidative addition yielding Ph3SbX2, 1.1;
- Ph3AsBr2 is an As(V) compound, whereas Ph3As.I2, Me3As.I2 and Me3As.Br2 are charge transfer complexes of the type shown in Figure 1.1d.
(1.1)
The nature of the products from reaction 1.3 are dependent on the solvent and the R group in R3P. Solid state structure determinations exemplify products of type [R3PI]+[I3]- (e.g. R = nPr2N, solvent = Et2O) and [(R3PI(2I3]+[I3]- (e.g. R = Ph, solvent = CH2Cl2; R = iPr, solvent = Et2O). Structure 1.2 shows the [(iPr3PI)2I3]+ cation in [(R3PI)2I3][I3].
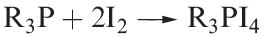 (1.3)
(1.3)
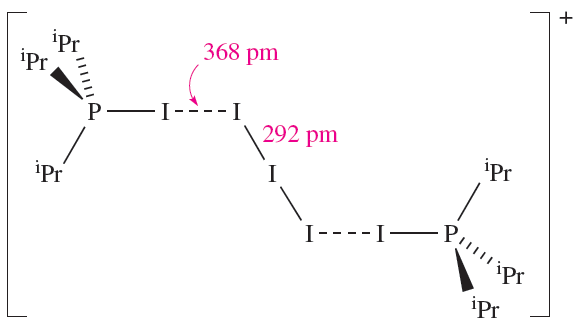
(1.2)
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


