

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Aluminium
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 511
2-3-2017
2862
Aluminium
Aluminium alkyls can be prepared by the transmetallation reaction 1.1, or from Grignard reagents (equation1.2). On an industrial scale, the direct reaction of Al with a terminal alkene and H2 (equation 1.3) is employed.
 (1.1)
(1.1)
 (1.2)
(1.2)
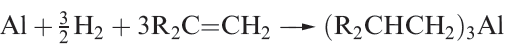 (1.3)
(1.3)
Reactions between Al and alkyl halides yield alkyl aluminium halides (equation 1.4); note that 18.7 is in equilibrium with [R2Al(µ-X)2AlR2] and [RXAl(µ-X(2AlRX] via a redistribution reaction, but the bridge Aluminium compound in below predominates in the mixture.
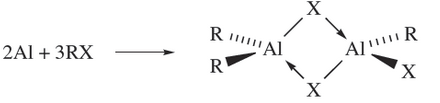 (1.4)
(1.4)
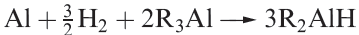 (1.5)
(1.5)
Alkyl aluminium hydrides are obtained by reaction 1.5. These compounds, although unstable to both air and water, are important catalysts for the polymerization of alkenes and other unsaturated organic compounds. Ziegler–Natta catalysts containing trialkyl aluminium compounds are introduced in Box 18.3.
 (1.1)
(1.1)
Earlier we noted that R3B compounds are monomeric. Trimethylaluminium (mp 313 K) possesses structure 1.1 and so bonding schemes can be developed in like manner as for B2H6. The fact that Al_Cbridge > Al_Cterminal is consistent with 3c-2e bonding in the Al_C_Al bridges, but with 2c-2e terminal bonds. Equilibria between dimer and monomer exist in solution, with the monomer becoming more favoured as the steric demands of the alkyl group increase. Mixed alkyl halides also dimerize as exemplified in structure 18.7, but with particularly bulky R groups, the monomer (with trigonal planar Al) is favoured, e.g.(2,4,6-tBu3C6H2)AlCl2 (Figure 1.1a).
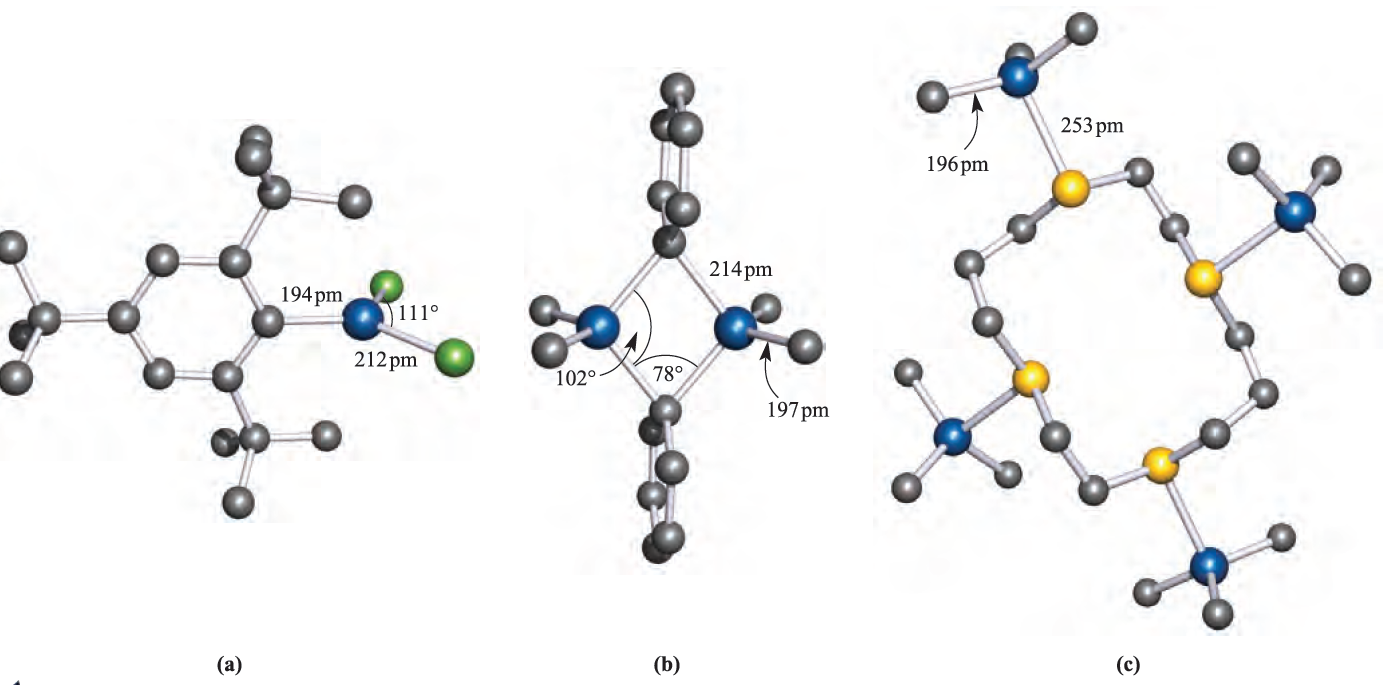
Fig. 1.1 The solid state structures (X-ray diffraction) of (a) (2,4,6-tBu3C6H2(AlCl2 [R.J. Wehmschulte et al. (1996) Inorg. Chem., vol. 35, p. 3262], (b) Me2Al)µ-Ph)2AlMe2 [J.F. Malone et al. (1972) J. Chem. Soc., Dalton Trans., p. 2649], and (c) the adduct L_ًAlMe3ق4 where L is the sulfur-containing macrocyclic ligand 1,4,8,11-tetrathiacyclotetradecane [G.H. Robinson et al. (1987) Organometallics, vol. 6, p. 887]. Hydrogen atoms are omitted for clarity; colour code: Al, blue; C, grey; Cl, green; S, yellow.
Triphenylaluminium also exists as a dimer, but in the mesityl derivative (mesityl=2,4,6-Me3C6H2), the steric demands of the substituents stabilize the monomer. Figure 1.1b shows the structure of Me2Al)µ-Ph(2AlMe2, and the orientations of the bridging phenyl groups are the same as in Ph2Al(µ-Ph)2AlPh2. This orientation is sterically favoured and places each ipso-carbon atom in an approximately tetrahedral environment. The ipso-carbon atom of a phenyl ring is the one to which the substituent is attached; e.g. in PPh3, the ipso-C of each Ph ring is bonded to P. In dimers containing RC≡C-bridges, a different type of bonding operates. The structure of Ph2Al)PhC≡C(2AlPh2 (1.2) shows that the alkynyl bridges lean over towards one of the Al centres. This is interpreted in terms of their behaving as σ ,π-ligands: each forms one Al_C σ-bond and interacts with the second Al centre by using the C≡C π- bond. Thus, each alkynyl group is able to provide three electrons for bridge bonding in contrast to one electron being supplied by an alkyl or aryl group; the bonding is shown schematically in 1.3.

(1.2) (1.3)
Trialkylaluminium derivatives behave as Lewis acids, forming a range of adducts, e.g. R3N.AlR3, K[AlR3F], Ph3P.AlMe3 and more exotic complexes such as that shown in Figure 18.8c. Each adduct contains a tetrahedrally sited Al atom. Trialkylaluminium compounds are stronger Lewis acids than either R3B or R3Ga, and the sequence for group 13 follows the trend R3B < R3Al > R3Ga > R3In > R3Tl.
The first R2Al_AlR2 derivative was reported in 1988, and was prepared by potassium reduction of the sterically hindered {(Me3Si)2CH}2AlCl. The Al_Al bond distance in {(Me3Si)2CH}4Al2 is 266pm (compare rcov = 130 pm) and the Al2C4 framework is planar, despite this being a singly bonded compound. A related compound is (2,4,6- iPr3C6H2)4Al2 (Al_Al = 265 pm) and here the Al2C4 framework is non-planar (angle between the two AlC2 planes = 458). One-electron reduction of Al2R4 (R = 2,4,6-iPr3C6H2) gives the radical anion [Al2R4]- with a formal Al_Al bond order of 1.5. Consistent with the presence of a π-contribution, the Al_Al bond is shortened upon reduction to 253pm for R = (Me3Si)2CH, and 247pm for R = 2,4,6-iPr3C6H2; in both anions, the Al2R4 frameworks are essentially planar. In theory, a dialane R2Al_AlR2, 1.4, possesses an isomer, 1.5, and such a species is exemplified by (η5-C5Me5)Al_Al(C6F5)3. The Al_Al bond (259 pm) in this compound is shorter than in compounds of type R2Al_AlR2 and this is consistent with the ionic contribution made to the Al_Al interaction in isomer 1.5.
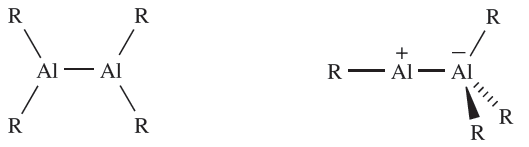
(1.4) (1.5) (1.6)
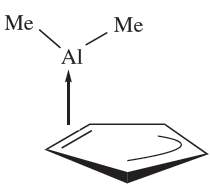
The reaction between cyclopentadiene and Al2Me6 gives CpAlMe2 which is a volatile solid. In the gas phase, it is monomeric with an η2-Cp bonding mode (1.6). This effectively partitions the cyclopentadienyl ring into alkene and allyl parts, since only two of the five π-electrons are donated to the metal centre. In the solid state, the molecules interact to form polymeric chains (Figure 1.2a).
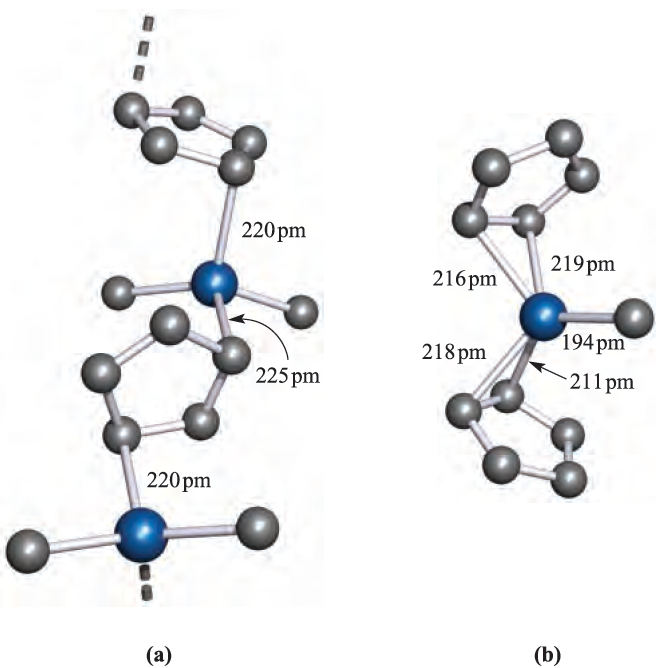
Fig. 1.2 The solid state structures (X-ray diffraction) of (a) polymeric CpAlMe2 [B. Tecle et al. (1982) Inorg. Chem., vol. 21, p. 458], and (b) monomeric(η2-Cp)2AlMe [J.D. Fisher et al. (1994) Organometallics, vol. 13, p. 3324]. Hydrogen atoms are omitted for clarity; colour code: Al, blue; C, grey.
The related compound Cp2AlMe is monomeric with an η2-mode in the solid state (Figure 1.2b). In solution, Cp2AlMe and CpAlMe2 are highly fluxional. A low energy difference between the different modes of bonding of the cyclopentadienyl ligand is also observed in the compounds (C5H5)3Al (i.e. Cp3Al),(1,2,4-Me3C5H2(3Al and )Me4C5H(3Al. In solution, even at low temperature, these are stereochemically non-rigid, with negligible energy differences between η1-, η2-, η3- and η5-modes of bonding. In the solid state, the structural parameters are consistent solid state, the structural parameters are consistent with the descriptions:

These examples serve to indicate the non-predictable nature of these systems, and that subtle balances of steric and electronic effects are in operation.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















