

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Stereoisomerism: optical isomers
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 549
28-2-2017
1373
Stereoisomerism: optical isomers
Optical isomerism is concerned with chirality, and some important terms relating to chiral complexes are defined in Box 19.2. The simplest case of optical isomerism among d-block complexes involves a metal ion surrounded by three didentate ligands, for example [Cr(acac)3] or [Co(en(3]3+ (fig. 1.1).
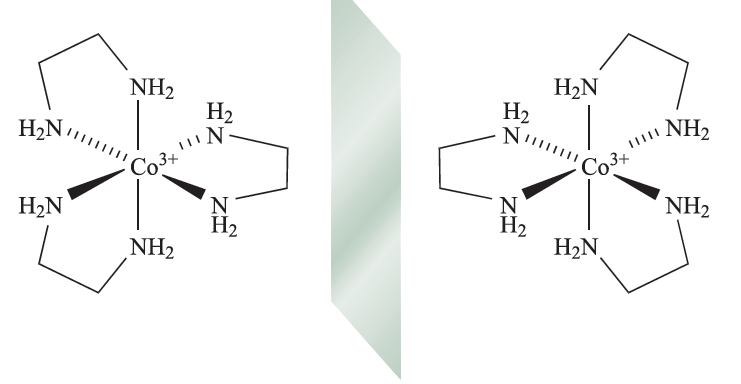
Fig. 1.1 The octahedral complex [Co(en)3]3+ contains three didentate ligands and therefore possesses optical isomers, i.e. the isomers are non-superposable mirror images of each other.
These are examples of tris-chelate complexes. Pairs of enantiomers such as Δ- and Λ-[Cr(acac)3] or Δ- and Λ-[Co(en)3]Cl3 differ only in their action on polarized light. However, for ionic complexes such as [Co(en)3]3+, there is the opportunity to form salts with a chiral counter-ion A-. These salts now contain two different types of chirality: the Δ- or Λ- chirality at the metal centre and the (+) or (_) chirality of the anion. Four combinations are possible of which the pair {Δ-(+)g and {Λ-(_)} is enantiomeric as is the pair {Δ-(_)} and {Λ-(+)} However, with a given anion chirality, the pair of salts {fΔ-(_)} and {Λ-(_)} are diastereomers (see Box 19.2) and may differ in the packing of the ions in the solid state, and separation by fractional crystallization is often possible. Bis-chelate octahedral complexes such as [Co(en)2Cl2]+ exist in both cis- and trans-isomers depending on the arrangement of the chloro ligands. In addition, the cis-isomer (but not the trans) possesses optical isomers. The first purely inorganic complex to be resolved into its optical isomers was [CoL3]6+ (1.1) in which each L ligand is the complex cis-[Co(NH3)4(OH(2]+ which chelates through the two Odonor atoms.
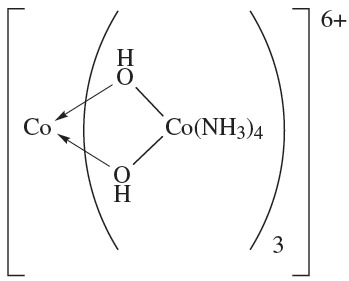
(1.1)
Chirality is not usually associated with square planar complexes but there are some special cases where chirality is introduced as a result of, for example, steric interactions between two ligands. In 1.2, steric repulsions between the two R groups may cause the aromatic substituents to twist so that the plane of each C6-ring is no longer orthogonal to the plane that defines the square planar environment around M.
Such a twist is defined by the torsion angle A–B–C–D, and renders the molecule chiral. The chirality can be recognized in terms of a handedness, as in a helix, and the terms P and M (see Box 19.2) can be used to distinguish between related chiralmolecules. If, in 1.2, the Cahn–Ingold–Prelog priority of R is higher than R’ (e.g. R = Me, R’ = H), then a positive torsion angle corresponds to P-chirality. An example is trans- [PdCl2(2-Mepy)2] (2-Mepy = 2-methylpyridine), for which the P-isomer shown in Figure 1.2.

(1.2)
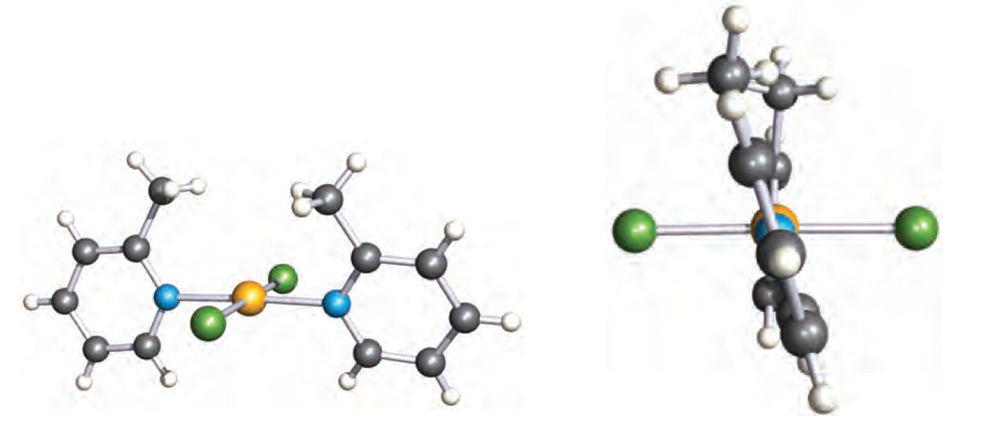
Fig. 1.2 Two views of the structure (X-ray diffraction) of trans-[PdCl2(2-Mepy)2] (2-Mepy = 2-methylpyridine) showing the square planar environment of the Pd(II) centre and the mutual twisting of the 2-methylpyridine ligands. The torsion angle between the rings is 18.68 [M.C. Biagini (1999) J. Chem. Soc., Dalton Trans., p. 1575]. Colour code: Pd, yellow; N, blue; Cl, green; C, grey; H, white.
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















