

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Thermodynamic aspects: the Irving–Williams series
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 588
22-2-2017
1574
Thermodynamic aspects: the Irving–Williams series
In aqueous solution, water is replaced by other ligands (equation 1.1) and the position of equilibrium will be related to the difference between two LFSEs, since Δoct is ligand-dependent.
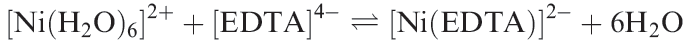 (1.1)
(1.1)
Table 1.1 lists overall stability constants for [M(en(3]2+ and [M(EDTA)]2- high-spin complexes for d5 to d10 first row M2+ ions. For a given ligand and cation charge, ΔSo should be nearly constant along the series and the variation in log βn should approximately parallel the trend in values of _ΔHo. Table 1.1 shows that the trend from d5 to d10 follows a ‘single hump’, with the ordering of log βn for the high-spin ions being: Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+
Table 1.1 Overall stability constants for selected high-spin d-block metal complexes.
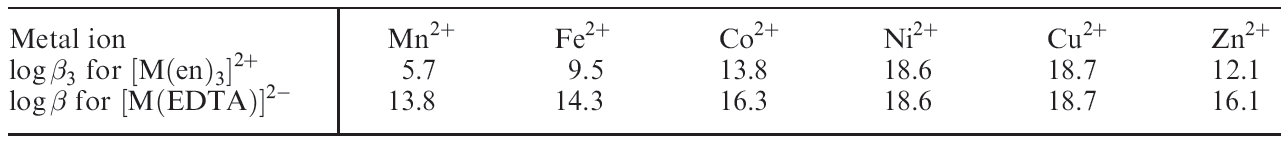
This is called the Irving–Williams series and is observed for a wide range of ligands. The trend is a ‘hump’ that peaks at Cu2+ (d9) and not at Ni2+ (d8) as might be expected from a consideration of LFSEs while the variation in LFSE values is a contributing factor, it is not the sole arbiter. Trends in stability constants should bear a relationship to trends in ionic radii (see Appendix 6); the pattern in values of rion for 6- coordinate high-spin ions is: Mn2+ > Fe2+ > Co2+ > Ni2+ < Cu2+ < Zn2+ We might expect rion to decrease from Mn2+ to Zn2+ as Zeff increases, but once again we see a dependence on the d n configuration with Ni2+ being smallest. In turn, this predicts the highest value of log βn for Ni2+. Why, then, are copper(II) complexes so much more stable than might be expected? The answer lies in the Jahn–Teller distortion that a d9 complex suffers; the six metal–ligand bonds are not of equal length and thus the concept of a ‘fixed’ ionic radius for Cu2+ is not valid. In an elongated complex such as [Cu(H2O)6]2+, there are four short and two long Cu_O bonds. Plots of stepwise stability constants for the displacement of H2O by NH3 ligands in [Cu(H2O)6]2+ and [Ni(H2O)6]2+ are shown in Figure 1.1; for the first four substitution steps, complex stability is greater for Cu2+ than Ni2+, reflecting the formation of four short (strong) Cu_N bonds. The value of logK5 for Cu2+ is consistent with the formation of a weak (axial) Cu_N bond; logK6 cannot be measured in aqueous solution.
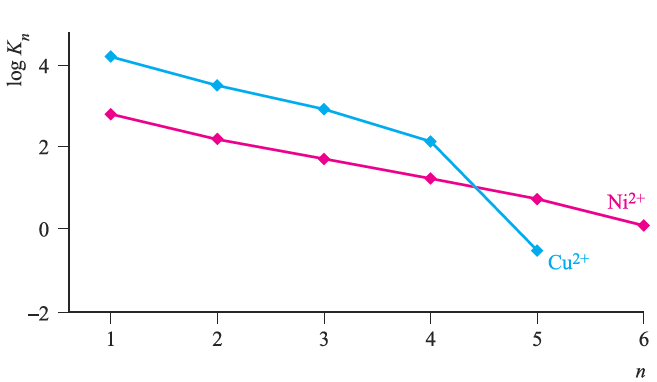
Fig. 1.1 Stepwise stability constants (logKn) for the displacement of H2O by NH3 from [Ni(H2O)6]2+ (d8) and [Cu)H2O)] 2+ (d9).
The magnitude of the overall stability constant for complexation of Cu2+ is dominated by values of Kn for the first four steps and the thermodynamic favourability of these displacement steps is responsible for the position of Cu2+ in the Irving–Williams series.
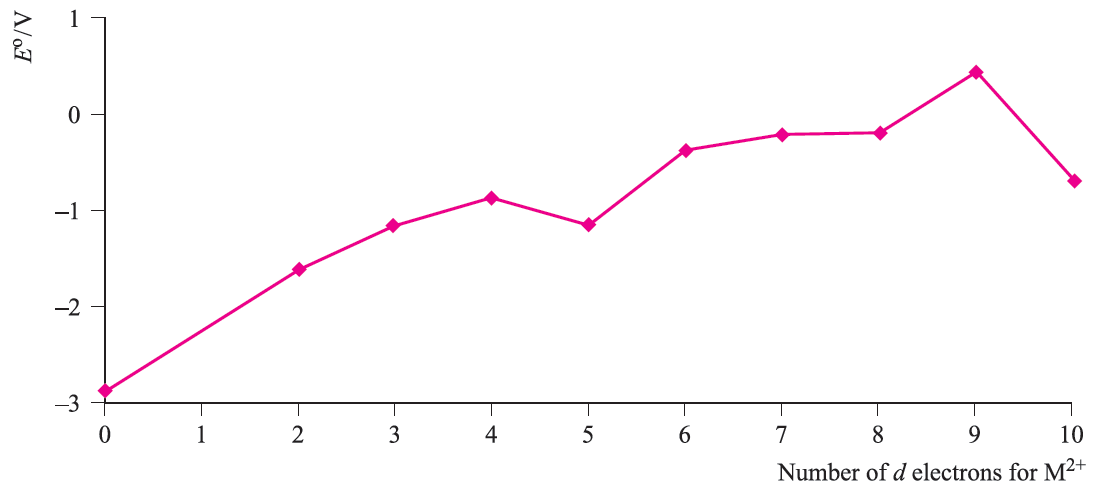
Fig. 1.2 The variation in values of Eo(M2+/M) as a function of d n configuration for the first row metals; the point for d0 corresponds to M = Ca.
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















