

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Phenolphthalein: Finding concentration with titration
المؤلف:
John T. Moore, EdD
المصدر:
Chemistry Essentials For Dummies
الجزء والصفحة:
p 156
26-1-2017
2113
Phenolphthalein: Finding concentration with titration
Phenolphthalein (pronounced fe-nul-tha-Leen) is a commonly used indicator. Phenolphthalein is
✓ Clear and colorless in an acid solution
✓ Pink in a basic solution
Chemists use phenolphthalein in a procedure called a titration, in which they determine the concentration of an acid or base by its reaction with a base or acid of known concentration. Here’s how to evaluate an acid solution using titration:
1. Add a couple drops of phenolphthalein to a known
volume of the acid solution you want to test.
Because you’re adding the indicator to an acidic solution, the solution in the flask remains clear and colorless. Suppose, for example, that you want to determine the molar concentration of an HCl solution. First, you place a known volume (say, 25.00 milliliters measured accurately with a pipette) in an Erlenmeyer flask (that’s just a flat-bottomed, conical-shaped flask) and add a couple drops of phenolphthalein solution.
2. Add small, measured amounts of a base of known molarity (concentration) until the solution turns light pink.
Add small amounts of a standardized sodium hydroxide (NaOH) solution of known molarity (for example, 0.100 M) with a buret. (A buret is a graduated glass tube with a small opening and a stopcock valve, which helps you measure precise volumes of solution.) Keep adding base until the solution turns the faintest shade of pink detectable. I call this the endpoint of the titration, the point at which the acid has been exactly neutralized by the base.
3. Write the balanced equation for the reaction.
Here’s the reaction:
HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq)
4. Calculate the molarity of the acidic solution.
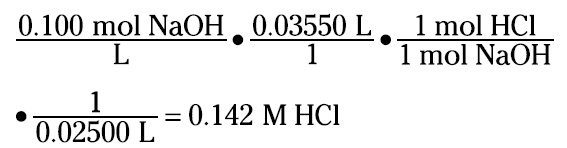
From the balanced equation, you can see that the acid and base react in a 1:1 mole ratio. So if you can calculate the moles of bases added, you’ll also know the number of moles of HCl present. Suppose that it takes 35.50 milliliters of the 0.100 M NaOH to reach the endpoint of the titration of the 25.00 milliliters of the HCl solution. Knowing the volume of the acid solution then allows you to calculate the molarity (note that you convert the milliliters to liters so that your units cancel nicely):
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















