
Reaction stoichiometry
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p 132
الجزء والصفحة:
p 132
 23-1-2017
23-1-2017
 2571
2571
Reaction stoichiometry
When you understand the weight relationships in a chemical reaction, you can do some stoichiometry problems. Stoichiometry refers to the mass relationship in chemical equations.
When you get ready to work stoichiometry types of problems, you must start with a balanced chemical equation. If you don’t have it to start with, go ahead and balance the equation.
Look at my favorite reaction — you guessed it — the Haber process:
N2(g) + 3 H2(g) ↔ 2 NH3(g)
Suppose that you want to know how many grams of ammonia can be produced from the reaction of 75.00 grams of nitrogen with excess hydrogen. The mole concept is the key. The coefficients in the balanced equation are not only the number of individual atoms or molecules but also the number of moles:
N2(g) + 3 H2(g) ↔ 2 NH3(g)
1 mole + 3 moles ↔ 2 moles
1 mol(28.014 g/mol) + 3 mol(2.016 g/mol) = 2 mol(17.031g/mol)
First, convert the 75.00 grams of nitrogen to moles of nitrogen. Then use the ratio of the moles of ammonia to the moles of nitrogen from the balanced equation to convert to moles of ammonia. Finally, take the moles of ammonia and number to grams. The equation looks like this:
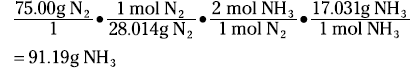
A stoichiometric ratio — such as mol NH3/mol N2 — enables you to convert from the moles of one substance in a balanced chemical equation to the moles of another substance.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


