
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Benzene Ring
المؤلف:
Franklin Potter and Christopher Jargodzki
المصدر:
Mad about Modern Physics
الجزء والصفحة:
p 69
20-10-2016
697
Benzene Ring
The benzene molecule is a ring of six carbon atoms, each C atom having one H atom attached. There is a mystery about the energy contained in this molecule. The benzene ring can be broken up into pieces, and chemists have measured the energies associated with the pieces and with the single bonds and the double bonds by studying ethylene and so on. The expected total energy can be calculated from these data, but the actual total energy of the benzene ring is much lower, telling us that the carbon atoms are much more tightly bound. Therefore, the bond picture would make the benzene ring easily susceptible to chemical attack, yet the molecule is quite resilient to breaking up.
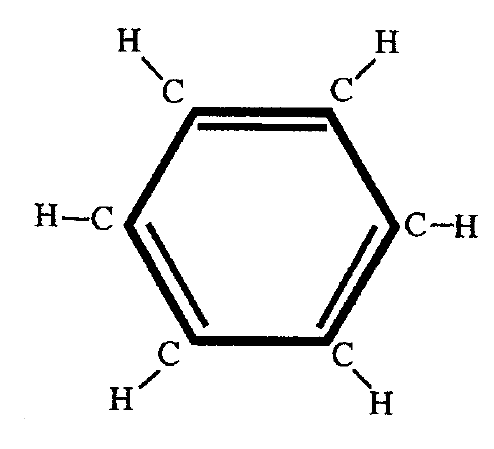
Using the Schrodinger equation by considering each carbon atom on this ring as the potential home for a single electron, one can calculate the possible energy levels for the benzene ring. Why does this method of calculation work?
Answer
The benzene ring has six-fold rotational symmetry about an axis perpendicular to the plane of the ring. One simply requires a wave function solution of the Schrodinger wave equation that has this six-fold symmetry, and such a solution is easy to find. One would expect that knowing this solution would allow one to calculate the energy levels.
However, we are not done! There are two possible configuration base states, as shown in the diagram.
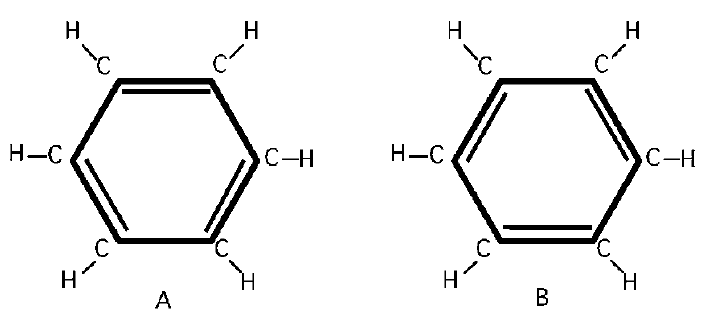
Both states should have the same energy, and they do. Therefore we really have a two-state system, analogous to the hydrogen molecular ion or the ammonia molecule, so the analysis should be for a two-state system. There will be the possibility that configuration A changes into configuration B. As a result, quantum mechanics will reveal that two new stationary states will occur, one state (the new ground state) with energy below the ground (lowest) state determined before, and one state with higher energy. The new ground state will be neither of the two configuration states shown but will be a linear combination of these two configuration states. Only this state is involved in the chemistry of benzene at room temperatures.
Understanding benzene was one of the first verifications of the linear superposition of states that is at the heart of quantum mechanics and also indicated that quantum mechanics will be successful at larger scales than atomic.
 الاكثر قراءة في طرائف الفيزياء
الاكثر قراءة في طرائف الفيزياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















