

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Linear Sequence in DNA Encodes Proteins with Three-Dimensional Structures
المؤلف:
David L. Nelson, Michael M. Cox
المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
الجزء والصفحة:
p 29
11-9-2016
2290
The Linear Sequence in DNA Encodes Proteins with Three-Dimensional Structures
The information in DNA is encoded in its linear (onedimensional) sequence of deoxyribonucleotide subunits, but the expression of this information results in a three-dimensional cell. This change from one to three dimensions occurs in two phases.
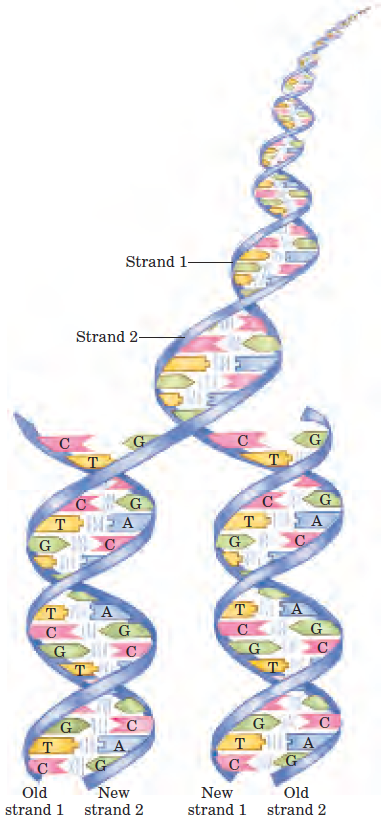
FIGURE 1–30 Complementarity between the two strands of DNA. DNA is a linear polymer of covalently joined deoxyribonucleotides, of four types: deoxyadenylate (A), deoxyguanylate (G), deoxycytidylate (C), and deoxythymidylate (T). Each nucleotide, with its unique three-dimensional structure, can associate very specifically but noncovalently with one other nucleotide in the complementary chain: A always associates with T, and G with C. Thus, in the double-stranded DNA molecule, the entire sequence of nucleotides in one strand is complementary to the sequence in the other. The two strands, held together by hydrogen bonds (represented here by vertical blue lines) between each pair of complementary nucleotides, twist about each other to form the DNA double helix. In DNA replication, the two strands separate and two new strands are synthesized, each with a sequence complementary to one of the original strands. The result is two double-helical molecules, each identical to the original DNA.
A linear sequence of deoxyribonucleotides in DNA codes (through an intermediary, RNA) for the production of a protein with a corresponding linear sequence of amino acids (Fig. 1–31). The protein folds into a particular three-dimensional shape, determined by its amino acid sequence and stabilized primarily by noncovalent interactions. Although the final shape of the folded protein is dictated by its amino acid sequence, the folding process is aided by
“molecular chaperones,” which catalyze the process by discouraging incorrect folding. The precise threedimensional structure, or native conformation, of the protein is crucial to its function. Once in its native conformation, a protein may associate noncovalently with other proteins, or with nucleic acids or lipids, to form supramolecular complexes such as chromosomes, ribosomes, and membranes. The individual molecules of these complexes have specific, high-affinity binding sites for each other, and within the cell they spontaneously form functional complexes.

FIGURE 1–31 DNA to RNA to protein. Linear sequences of deoxyribonucleotides in DNA, arranged into units known as genes, are transcribed into ribonucleic acid (RNA) molecules with complementary ribonucleotide sequences. The RNA sequences are then translated into linear protein chains, which fold into their native three-dimensional shapes, often aided by molecular chaperones. Individual proteins commonly associate with other proteins to form supramolecular complexes, stabilized by numerous weak interactions.
Although protein sequences carry all necessary information for the folding into their native conformation, this correct folding requires the right environment—pH, ionic strength, metal ion concentrations, and so forth. Self-assembly therefore requires both information (provided by the DNA sequence) and environment (the interior of a living cell), and in this sense the DNA sequence alone is not enough to dictate the formation of a cell. As Rudolph Virchow, the nineteenth-century Prussian pathologist and researcher, concluded, “Omnis cellula e cellula”: every cell comes from another cell.
 الاكثر قراءة في الاحماض النووية
الاكثر قراءة في الاحماض النووية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















