

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Complexes with metal–ligand π-bonding
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 566
23-8-2016
2676
Complexes with metal–ligand π-bonding
The metal dxy, dyz and dxz atomic orbitals (the t2g set) are nonbonding in an [ML6]n+, σ-bonded complex (Figure 1.1) and these orbitals may overlap with ligand orbitals of the correct symmetry to give π-interactions (Figure 1.2).
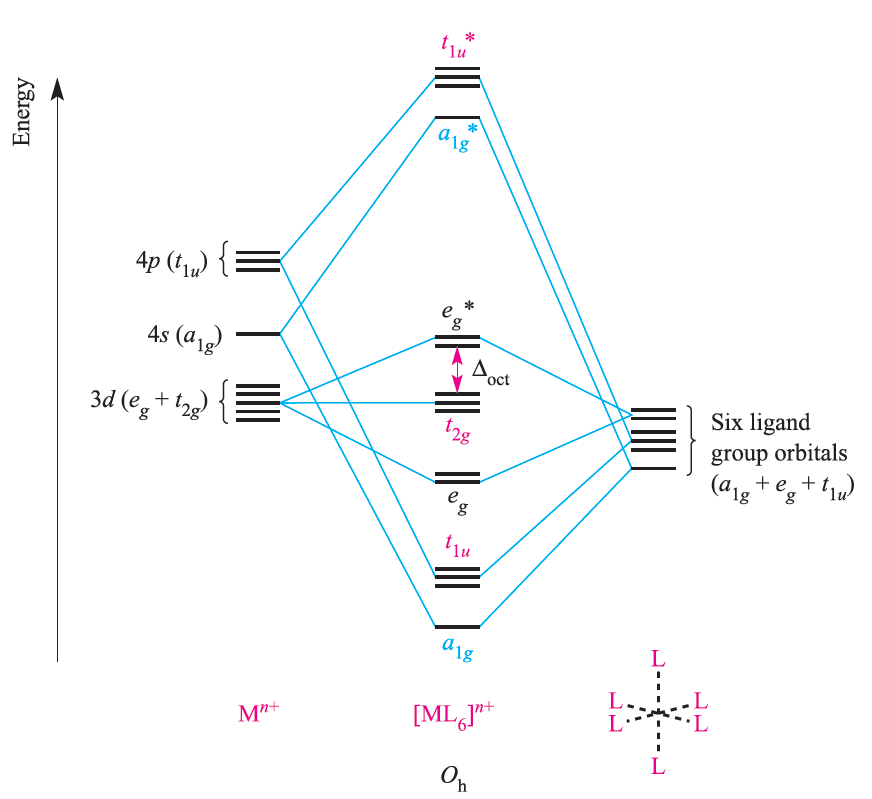
Fig. 1.1 An approximate MO diagram for the formation of [ML6]n+ (where M is a first row metal) using the ligand group orbital approach. The bonding only involves M_L π-interactions.
Although π-bonding between metal and ligand d orbitals is sometimes considered for interactions between metals and phosphine ligands (e.g. PR3 or PF3), it is more realistic to consider the roles of ligand σ*-orbitals as the acceptor orbitals. Two types of ligand must be differentiated: π-donor and π-acceptor ligands.
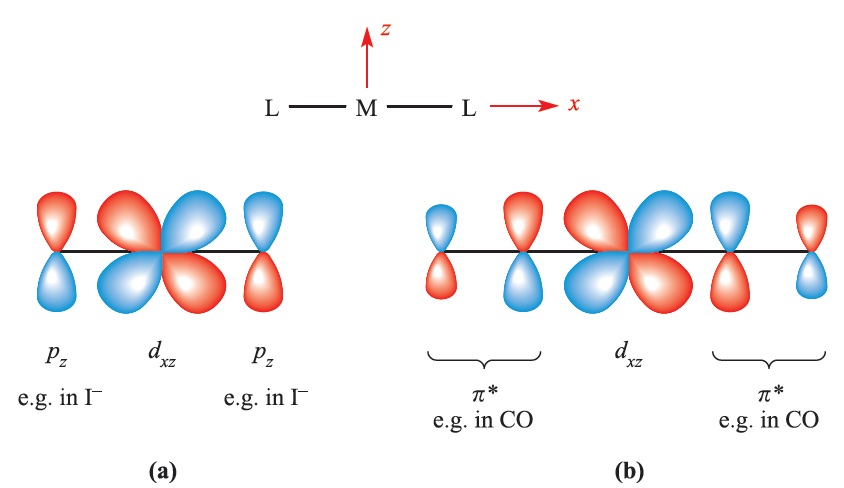
Fig. 1.2 π-Bond formation in a linear L_M_L unit in which the metal and ligand donor atoms lie on the x axis: (a) between metal dxz and ligand pz orbitals as for L = I-, an example of a π-donor ligand; and (b) between metal dxz and ligand π*-orbitals as for L = CO, an example of a π-acceptor ligand.
A π-donor ligand donates electrons to the metal centre in an interaction that involves a filled ligand orbital and an empty metal orbital; a π-acceptor ligand accepts electrons from the metal centre in an interaction that involves a filled metal orbital and an empty ligand orbital. π-Donor ligands include Cl-, Br- and I- and the metal– ligand π-interaction involves transfer of electrons from filled ligand p orbitals to the metal centre (Figure 1.2a). Examples of π-acceptor ligands are CO, N2, NO and alkenes, and the metal–ligand π-bonds arise from the back donation of electrons from the metal centre to vacant antibonding orbitals on the ligand (for example, Figure 1.2b). π-Acceptor ligands can stabilize low oxidation state metal complexes. Figure 1.3 shows partial MO diagrams which describe metal–ligand π- interactions in octahedral complexes; the metal s and p orbitals which are involved in σ-bonding have been omitted. Figure 20.14a shows the interaction between a metal ion and six π-donor ligands; electrons are omitted from the diagram, and we return to them later. The ligand group π-orbitals are filled and lie above, but relatively close to, the ligand σ-orbitals, and interaction with the metal dxy, dyz and dxz atomic orbitals leads to bonding (t2g) and antibonding (t2g*) MOs. The energy separation between the t2g* and eg* levels corresponds to Δoct. Figure 1.3b shows the interaction between a metal ion and six π-acceptor ligands. The vacant ligand π* orbitals lie significantly higher in energy than the ligand σ-orbitals. Orbital interaction leads to bonding (t2g) and antibonding (t2g*) MOs as before, but now the t2g* MOs are at high energy and Δoct is identified as the energy separation between the t2g and eg* levels (Figure 1.3b).



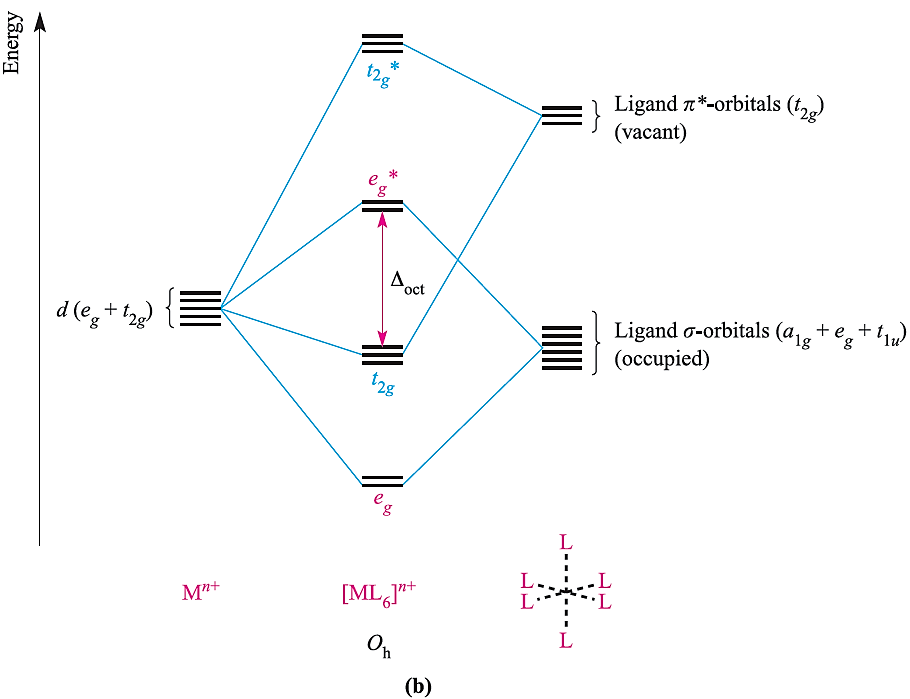
Fig. 1.3 Approximate partial MO diagrams for metal–ligand π-bonding in an octahedral complex: (a) with π-donor ligands and (b) with π-acceptor ligands. In addition to the MOs shown, σ-bonding in the complex involves the a1g and t1u MOs . Electrons are omitted from the diagram, because we are dealing with a general Mn+ ion. Compared with Figure 1.1, the energy scale is expanded.
Although Figures 1.1 and 1.3 are qualitative, they reveal important differences between octahedral [ML6]n+ complexes containing σ-donor, π-donor and π-acceptor ligands:
- Δoct decreases in going from a σ-complex to one containing π-donor ligands;
- for a complex with π-donor ligands, increased π-donation stabilizes the t2g level and destabilizes the t2g*, thus decreasing Δoct;
- Δoct values are relatively large for complexes containing π-acceptor ligands, and such complexes are likely to be low-spin;
- for a complex with π-acceptor ligands, increased π-acceptance stabilizes the t2g level, increasing Δoct.
The above points are consistent with the positions of the ligands in the spectrochemical series; π-donors such as I- and Br- are weak-field, while π-acceptor ligands such as CO and [CN]- are strong-field ligands. Let us complete this section by considering the occupancies of the MOs in Figures 1.3a and 1.3b. Six π-donor ligands provide 18 electrons (12 σ- and six π-electrons) and these can notionally be considered to occupy the a1g, t1u, eg and t2g orbitals of the complex. The occupancy of the t2g* and eg* levels corresponds to the number of valence electrons of the metal ion. Six π-acceptor ligands provide 12 electrons (the π-ligand orbitals are empty) and, formally, we can place these in the a1g, t1u and eg orbitals of the complex. The number of electrons supplied by the metal centre then corresponds to the occupancy of the t2g and eg* levels. Since occupying antibonding MOs is detrimental to metal–ligand bond formation, it follows that, for example, octahedral complexes with π-accepting ligands will not be favoured for metal centres with d7, d8, d9 or d10 configurations.
This last point brings us to back to some fundamental observations in experimental inorganic chemistry: d-block metal organometallic and related complexes tend to obey the effective atomic number rule or 18-electron rule.
A low oxidation state organometallic complex contains π-acceptor ligands and the metal centre tends to acquire 18 electrons in its valence shell (the 18 electron rule), thus filling the valence orbitals, e.g. Cr in Cr(CO)6, Fe in Fe(CO)5, and Ni in Ni(CO)4.
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















