

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Electronegativity and Size of Atoms and Acid/Base Strength
المؤلف:
ADAM RENSLO
المصدر:
the organic chemistry of medicinal agent
الجزء والصفحة:
p255
12-7-2016
4396
Electronegativity and Size of Atoms and Acid/Base Strength
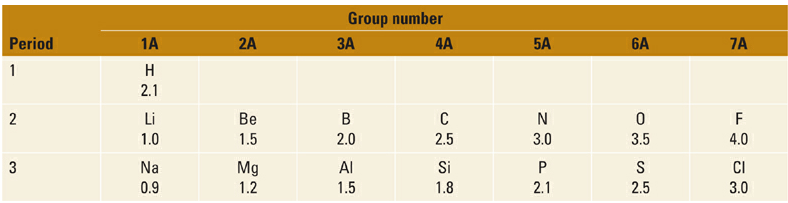
Two intrinsic properties of atoms affect the strengths of X–H bonds independent of other structural features. The first of these is the electronegativity of X, which can be compared for elements within the same period (i.e., row of the periodic table) that use the same valence shell electrons to form bonds (Table 1.1).
Table 1.1 Electronegativities of Elements in the First Three Rows of the Periodc Tiable.
The effect of electronegativity on acidity can be seen in the acidities (pKa values) of the simple compounds CH4, NH3, H2O, and HF, based on elements from the second row of the periodic table (Figure 1.1).
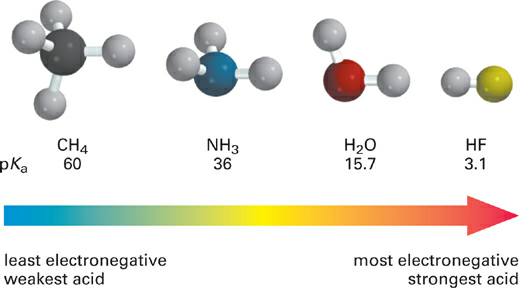
Figure 1.1 Example of effect of electronegativity on the acidity of X–H bonds in similar molecular contexts. (Reproduced, with permission, from Carey FA, Giuliano RM. Organic Chemistry. 9th ed. New York: McGraw-Hill Education; 2014.)
As the electronegativity of the X: atom increases in this series, the polarity of the X–H bond increases so that the H carries a larger partial positive charge (δ− X–H δ+) and the equilibrium for proton transfer to water increases (the acid becomes stronger). Another way to look at this series is that as the X atom becomes more electronegative, it can stabilize negative charge better and thus let go of a proton more easily. With regard to the conjugate base :X−, we would say that the more electronegative the atom X, the weaker the base :X−. So comparing across rows, increasing electronegativity of the X atom increases acidity of X–H and decreases basicity of :X−.
The trend also holds for acids of the BH+ type as seen in the following example where the more electronegative oxygen is much less basic than nitrogen in a similar context (Figure 1.2).
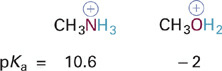
Figure 1.2 Effects of electronegativity on the acidity of +X–H bonds in similar molecular contexts (elements in the same row of the periodic table).
The negative pKa value indicates that protonated methanol (CH3OH2+) is quite a strong acid and therefore that neutral O: in methanol (CH3OH) must be a very weak base. By contrast, the pKa of methyl ammonium ion (CH3NH3+) indicates that it is a very weak acid and therefore that neutral N: in methylamine (CH3NH2) must be a reasonably strong base. Thus, the relative electronegativities of N and O correctly predict that neutral N: will be a better base than neutral O: and that CH3OH2+ is a stronger acid than CH3NH3+. Note however that such predictions will hold only when the atoms being compared are in the samecharge state, as is the case in the comparisons shown in Figures 1.2 and 1.2.
The second intrinsic property of elements that influences the acidity of their X–H bonds is their size. Elements within the same group (i.e., column) in the periodic table have the same number of valence shell electrons, but increase in size as we move down the table. As the atomic size increases, the X–H bond becomes longer, which weakens the bond and increases its acidity (Figure 1.3).
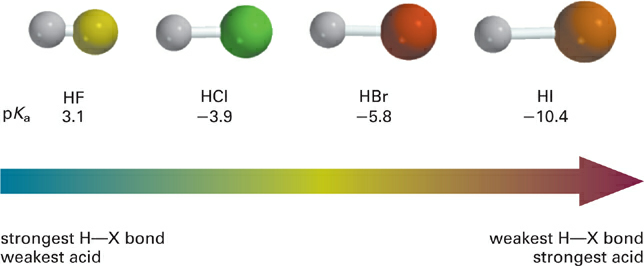
Figure 1.3 Example of effect of the size of the X atom on the acidity of X–H bonds in similar molecular contexts.
From the point of view of the conjugate base :X−, the negative charge becomes increasingly spread out as the size (i.e., volume) of the X atom increases. In general, as the negative charge in the conjugate base becomes more spread out, it becomes better stabilized and thus basicity decreases. So comparing down columns, increasing size of the X atom increases acidity of X–H and decreases basicity of :X−. As illustrated below, this trend also holds for acids of the BH+ type (Figure 1.4).
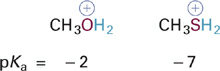
Figure 1.4 Example of the effects of atom size X on the acidity of +X–H bonds in similar molecular contexts (elements in the same column of the periodic table).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















