

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Three Theories of Acids and Bases
المؤلف:
ADAM RENSLO
المصدر:
the organic chemistry of medicinal agent
الجزء والصفحة:
p 236
23-6-2016
3756
Three Theories of Acids and Bases
In the late 1800s, Arrhenius proposed a theory of acids and bases based on observations of “what happens” when a substance is dissolved in pure water. The key observation was that some substances cause an increase in the hydrogen ion concentration, [H+], when dissolved in water and others cause an increase in the hydroxide concentration, [OH−]. On this basis, Arrhenius defined an acid as a compound that dissociates in water to give H+ and an anion (e.g., HCl → H+ + Cl−), and a base as a compound that dissociates in water to give a cation and OH− (e.g., KOH → K+ + OH−). Although the noted changes in [H+] and [OH−] can be a useful description of acid/base behavior for certain systems, the model is limited in the scope of acids/bases and solvents (water only) that can be described.
A more general theory of acids and bases that focuses specifically on “who has the proton” was proposed separately in 1923 by Brønsted in Denmark and by Lowry in England. In this model, the proton never exists in solution as an isolated ion because it is energetically too unstable. Instead it is always covalently bonded to another atom, but can be transferred between lone pairs of electrons on two different atoms. In this proton transferreaction, the proton donor is the Brønsted–Lowry acid and the proton acceptor, that is, the atom (or atom within a molecule) that takes the proton from the acid, is the Brønsted–Lowry base (Figure 1.1). The Brønsted–Lowry model of acids and bases is the foundation for our discussions in this chapter.
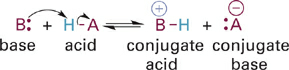
Figure 1.1 Proton transfer reaction between a Brønsted–Lowry acid and base.
In a typical Brønsted–Lowry acid-base reaction (Figure 1.1) the initial acid (HA) is converted to its conjugate base (:A−), and the initial base (B:) is concomitantly converted to its conjugate acid (BH+). Significantly, this approach to acid/base chemistry focuses attention on the molecular structures of the two bases that transfer H+ between them (B: and :A− in Figure 1.1) allowing a direct comparison of the relationship of structure to basicity. Furthermore, since the acid and base forms of the same molecule (e.g., H–A and :A−) differ by only a single proton (H+), analysis of trends in acidity or basicity as a function of molecular structure provides significant insight into the relationship of structure to reactivity.
The Brønsted–Lowry definition is much broader in scope and works for different solvents, since H+ can be transferred between bases in any solvent. However, the behavior that Arrhenius observed for bases and acids in water is also consistent with the Brønsted–Lowry definition, in the sense that water (H2O) can act as either a base to accept a proton from another acid or as an acid to donate a proton to a base (Figure 1.2). Molecules like water that can act as both an acid and a base are called amphoteric molecules. Conceptually, the only difference between the models for the reaction of a neutral HA acid in water is that the proton is transferred to a water molecule to produce a hydronium ion rather than simply dissociating to H+. Note that only one of the two lone pairs on oxygen is shown in the acid-base reaction involving water (Figure 1.2). For simplicity’s sake, we will generally show only the lone pair involved in the acid-base reaction in the schemes and tables of this chapter.
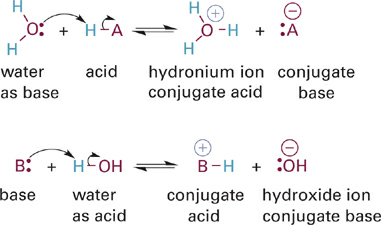
Figure 1.2 Reactions showing the relationship between the Brønsted–Lowry and Arrhenius definitions of acids and bases. Only those lone pair electrons involved in the acid-base reaction are shown explicitly.
A completely separate notion of acids and bases was devised by G. N. Lewis in 1923 and formally proposed in 1938. Rather than focusing only on protons, Lewis’ theory asks “who has the electrons?”. Thus, a Lewis acid is an electron acceptor and a Lewis base is an electron donor. With this definition, any atom that has a positive charge, a partial positive charge, or an unfilled valence shell of electrons is considered a Lewis acid. Metal ions such as Zn2+, Ca2+, and Mg2+ are common positively charged Lewis acids of importance in biological systems. Carbons found in highly polar bonds such as in a carbonyl group (δ+ C=O δ−) are important Lewis acids involved in many important biological reactions. Since boron has only three valence shell electrons of its own, compounds like BF3 are two electrons short of an octet, and thus are very strong Lewis acids (if not biologically relevant ones). Essentially any Brønsted base can also be considered a Lewis base since both utilize a lone pair of electrons to react with their corresponding acids (Figure 1.3).
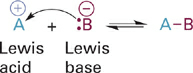
Figure 1.3 Reaction illustrating Lewis theory definition of acids and bases.
Note that whereas a Brønsted acid gives up its proton to a different Brønsted base when they react, a Lewis acid combines (i.e., forms a bond) with a Lewis base when they react (Figure 1.3).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















