

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
MO theory: heteronuclear diatomic molecules
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
p41
30-5-2016
5715
MO theory: heteronuclear diatomic molecules
In this section, we return to MO theory and apply it to hetero nuclear diatomic molecules. In each of the orbital interaction diagrams constructed for homonuclear diatomics, the resultant MOs contained equal contributions from each atomic orbital involved. This is represented in equation below for the bonding MO in H2 by the fact that each of the wavefunctions ψ1 and ψ2 contributes equally to MO, and the representations of the MOs in H2 depict symmetrical orbitals. Now we look at representative examples of diatomics in which the MOs may contain different atomic orbital contributions, a scenario that is typical for heteronuclear diatomics.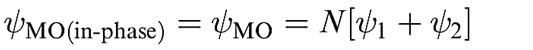
 (1.1)
(1.1)
First, we must consider likely restrictions when we are faced with the possibility of combining different types of atomic orbitals. Which orbital interactions should be considered?. We stated that orbital interactions are allowed if the symmetries of the atomic orbitals are compatible with one another. In our approach to the bonding in a diatomic, we made the assumption that only the interactions between like atomic orbitals, e.g. 2s–2s, 2pz–2pz, need be considered. Such interactions are symmetryallowed, and in addition, in a homonuclear diatomic the energies of like atomic orbitals on the two atoms are exactly matched.
In a heteronuclear diatomic, we often encounter two atoms that have different basis sets of atomic orbitals, or have sets of similar atomic orbitals lying at different energies. For example, in CO, although both C and O possess valence 2s and 2p atomic orbitals, the greater effective nuclear charge of O means that its atomic orbitals lie at a lower energy than those of C. Before we look more closely at some examples of heteronuclear diatomics, let us briefly consider some symmetry-allowed and disallowed orbital interactions. It is important to remember that we are looking at these symmetry properties with respect to the internuclear axis. In our earlier discussion of homonuclear diatomics, we ignored the possibility of overlap between the px and py orbitals. Such an interaction between orthogonal p atomic orbitals (Figure 1.1a) would give a zero overlap integral. Similarly, for nuclei lying on the z axis, interaction between px and pz, or py and pz, orbitals gives zero overlap. An interaction between an s and a p atomic orbital may occur depending upon the orientation of the p orbital. In Figure 1.1b, overlap would be partly bonding and partly antibonding and the net effect is a non-bonding interaction. On the other hand, Figure 1.1 c shows an s–p interaction that is allowed by symmetry.

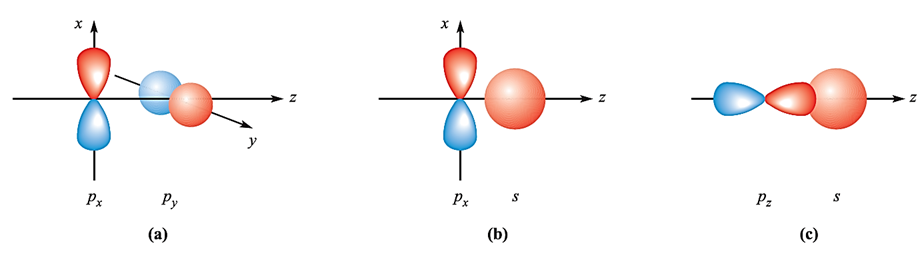
Fig. 1.1 Overlap between atomic orbitals is not always allowed by symmetry. Combinations (a) and (b) lead to non-bonding situations but (c) is symmetry-allowed and gives rise to a bonding interaction.


Fig. 1.2 The relative energies of atomic orbitals of X and Y will dictate whether an interaction (formally allowed by symmetry) will lead to efficient overlap or not. Here, an interaction occurs but the contribution made by Y to ψMO is greater than that made by ψX, while ψX contributes more than ψY to the antibonding MO. The diagrams on the right give pictorial representations of the bonding and antibonding MOs.
Whether or not this leads to effective overlap depends upon the relative energies of the two atomic orbitals. This is illustrated in Figure 1.2 for a diatomic XY. Let the interaction between ψX and ψY be symmetry-allowed; the orbital energies are not the same but are close enough that overlap between the orbitals is efficient. The orbital interaction diagram shows that the energy of the bonding MO is closer to E(ψY) than to E)ψX( and the consequence of this is that the bonding orbital possesses greater Y than X character. This is expressed in equation 1.2 in which c2 > c1. For the antibonding MO, the situation is reversed, and ψX contributes more than ψY; in equation 1.3, c3 > c4.
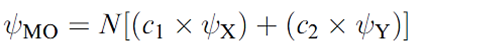
 (1.2)
(1.2)
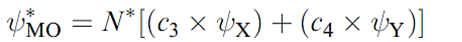
 (1.3)
(1.3)
The energy separation ΔE in Figure 1.2 is critical. If it is large, interaction between ψX and ψY will be inefficient (the overlap integral is very small). In the extreme case, there is no interaction at all and both ψX and ψY appear in the XY molecule as unperturbed non-bonding atomic orbitals. We illustrate this below.
Hydrogen fluoride
The ground state configurations of H and F are 1s and [He]2s2 2p5 respectively. Since Zeff(F) ˃Zeff(H), the F 2s and 2p atomic orbital energies are significantly lowered with respect to the H 1s atomic orbital (Figure 1.3). We now have to consider which atomic orbital interactions aresymmetry-allowed and then ask whether the atomic orbitals are sufficiently well energy-matched. First, define the axis set for the orbitals; let the nuclei lie on the z axis. Overlap between the H 1s and F 2s orbitals is allowed by symmetry, but the energy separation is very large (note the break on the energy axis in Figure 1.3).
Overlap between the H 1s and F 2pz atomic orbitals is also symmetry-allowed and there is a reasonable orbital energy match. As Figure 1.3 shows, an interaction occurs leading to σ and σ* MOs; the σ-orbital has greater F than H character. Notice that, because HF is non-centrosymmetric the symmetry labels of the orbitals for HF do not involve g and u labels. The two F 2px and 2py atomic orbitals become non-bonding orbitals in HF since no net bonding interaction with the H 1s atomic orbital is possible. Once the orbital interaction diagram has been constructed, the eight valence electrons are accommodated as shown in Figure 1.3, giving a bond order of 1 in HF. The MO picture of HF indicates that the electron density is greater around the F than H nucleus; the model is consistent with a polar H__F bond in the sense Hδ+__Fδ-. [Exercise: Sketch pictorial representations of the σ and σ* MOs in HF. Hint: Refer to Figure 1.2.]


Fig. 1.3 An orbital interaction diagram for the formation of HF. Only the valence atomic orbitals and electrons are shown. The break in the vertical (energy) axis indicates that the energy of the F 2s atomic orbital is much lower than is actually shown.
 الاكثر قراءة في نظريات التآصر الكيميائي
الاكثر قراءة في نظريات التآصر الكيميائي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















