

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Isoelectric focusing
المؤلف:
Clive Dennison
المصدر:
A guide to protein isolation
الجزء والصفحة:
20-4-2016
4230
Isoelectric focusing
Of the separation methods based upon gross physical properties such as charge or size etc., isoelectric focusing, is one of the most discriminating. Only methods based upon some biological property, which requires a subtle stereospecific relationship, are more discriminating. Sometimes IEF can almost be too discriminating because multiple bands can be obtained from what is essentially the same glycoprotein species, with only small differences in glycosylation a phenomenon known as “micro -heterogeneity”. A downside of IEF is that it is a relatively expensive, because of the cost of the ampholytes required.
Proteins are ampholytes having a pH-dependent net charge, which is positive at pH values below the proteins PI and negative at pH values above the PI.
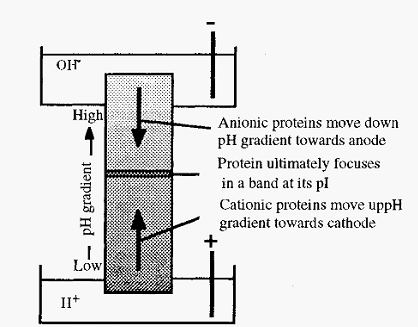
Figure 1. Schematic view of isoelectric focusing.
If proteins are distributed throughout a solution in a pH gradient, then upon application of an electrical potential across the gradient, with the anode at the low pH end, molecules in the low pH zone (which will be positively charged) will migrate to the cathode. Their migration will take them through zones of increasing pH, as a consequence of which they will gradually lose their positive charge and their rate of migration will slow
down. Conversely, molecules in the high pH zone will be negatively charged and will consequently migrate, through zones of decreasing pH, towards the anode. When each protein reaches a position where the pH is equal to its pI, it will lose all of its charge and its migration will cease. After a sufficient time, therefore, the respective molecules will in consequence become “focused” at their isoelectric points. In this way, a mixture of proteins can be separated, as each will focus at a characteristic pI, Furthermore, the pI values can readily be measured in this way. In practice, three difficulties must be overcome:
• A stable, uniform pH gradient must be established and maintained.
• The system must be stabilized against disturbances due to convection.
• A system must be devised for the measurement of the pH gradient and for determination of the positions of the focused bands.
1- Establishing a pH gradient
If a pure ampholyte, such as a protein, is added to pure water, the water will acquire a pH equal to the isoionic point of the ampholyte which for most practical purposes is the same as the pI of the ampholyte18. So, a stack of ampholytes of increasing pI, arranged one on top of the other, would constitute a pH gradient. Electrophoretic mobility is also a function of pI and, as has been outlined in the discussion of isotachophoresis , it is possible to electrophoretically stack ampholytes in order of their mobilities. However, in isotachophoresis a buffer is present to control the pH. If there were no buffer present, except the ampholytes, then in arranging themselves in order of mobility they would simultaneously generate a pH gradient, the pH at each point corresponding to the pI of the ampholyte at that point. Since each ampholyte would finally be at its pI, where it has no net charge, there should theoretically be no net movement of the pH gradient.
If the ampholytes making up the pH gradient were proteins, the gradient would have a few steps (as many as there are proteins), but these steps would tend to be quite large (Fig. 2). However, if synthetic, randomly substituted, polyamino-polycarboxylic acid ampholytes were used, then there would be a very large number of very small steps, which in effect gives a smooth pH gradient. A protein introduced into such a gradient will cause a plateau to be formed at its pI, the length being proportional to the amount of protein (Fig. 3).
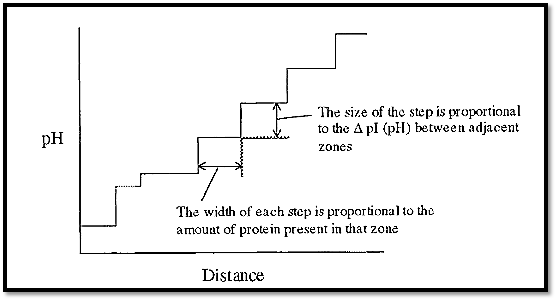
Figure 2. A pH gradient constructed from a stack of seven proteins.
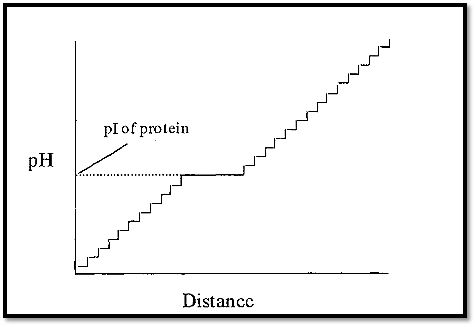
Figure 3. A pH gradient constructed from randomly synthesized polyamino-polycarboxylic acid ampholytes (and containing one protein).
The anode solution is acidic and the cathode solution is basic. Consequently, ampholyte molecules immediately in contact with the anode solution will be positively charged and those in contact with the cathode solution will be negatively charged. At zero time the pH distribution across the apparatus will be low at the anode end and high at the cathode end, but with a central plateau, corresponding to the pH of the sample plus mixed ampholytes (Fig. 4). When the electrical potential is applied across the electrodes, ampholytes will move to the anode or cathode, depending upon their charge, until they reach a pH at which they will have zero charge. Each ampholyte species will establish the pH at its pI and so with time the ampholytes will arrange themselves into an order of charge and in doing so will establish a smooth pH gradient (Fig. 4).
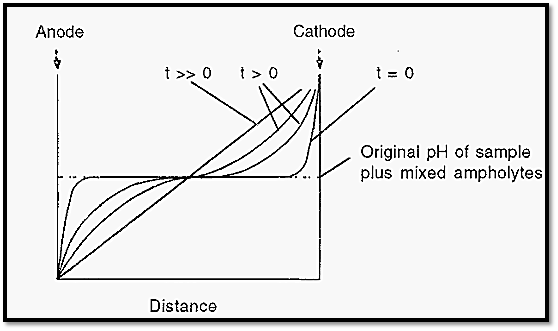
Figure 4. Time course of the establishment of a pH gradient in IEF.
Sample proteins added into this gradient will participate as ampholyte species and each will focus at its particular pI, introducing a small plateau in the gradient. Generally, it is desirable that samples are added in a way that avoids their exposure to the extreme pH values near the electrode solutions. This consideration has made open, flat bed systems popular as the pH gradient can be established and the samples can subsequently be applied at a position away from the electrodes.
One of the reasons for the discriminating power of IEF is that the principle of its operation intrinsically counteracts the effects of diffusion, which in other methods is responsible for the broadening of bands with time. Any protein which diffuses out of a focused band will enter a region of different pH where it will acquire a charge. In consequence it will immediately experience an electrophoretic force tending to move it back into the focused band. In this way the band is kept focused.
The steepness of the pH gradient, and its consequent resolving power, can be altered by using ampholytes covering different pH ranges. The range pH 3-10 is used first to get an overview of where the proteins focus and an appropriate choice of ampholytes can be made for a second round of focusing over a smaller range, say pH 5 to 7. The smaller the pH range covered, the greater will be the resolution.
2- Control of convection
Convective disturbances are currents induced in fluids by the effects of gravity upon fluids which differ in density in different parts. In IEF they are caused by heating effects, which reduces the density of the heated solution, and from the fact that focused protein bands are more dense than the surrounding ampholyte solutions. Practical systems consequently require some way of obviating these convective disturbances.
Since convection depends upon differences in density, the effect of gravity and a consequent movement of fluid, to avoid convection any one or more of these three elements could be targeted. Thus, an early approach was to conduct IEF in a sucrose gradient, thereby pre-imposing a density gradient which would damp out any convective disturbances.
To eliminate the effects of gravity, IEF may be conducted in a rotating tube, so that the gravity vectors cancel out with time, or, at somewhat greater expense, the experiment may be conducted under microgravity conditions in outer space. Finally, because convective disturbances are a consequence of fluid movement, another approach is to have a system where the liquid cannot move, such as in a gel, and a popular modern method is to conduct IEF in a gel slab.
3- Applying the sample and measuring the pH gradient
In any practical system, all of the attendant problems must be solved simultaneously. Thus, in addition to controlling convection, the system must allow for sample application - avoiding the pH extremes - and for measurement of the pH gradient after the separation. Different systems are suitable for analytical and preparative purposes and one of each will be briefly described, by way of illustration.
3.1 An analytical IEF system
The most common analytical system in use at present is the thin slab gel system. In this a thin layer of gel - either large pore polyacrylamide or agarose - containing an appropriate ampholyte mixture, is cast onto a backing sheet of Gel-Bond Æ, a product of FMC Inc. The sheet is positioned on top of a template on a cooling block. The template marks sample tracks, and serves as a guide to the application of the sample
Wicks, made of several layers of filter paper, are impregnated with the appropriate acid or base electrode solution and placed on top of the gel at each end (Fig. 5). When the apparatus is sealed, electrodes contact the filter paper wicks, thereby closing the electrical circuit (this description is based on the Pharmacia Biotech Multiphor apparatus).
The pH gradient is allowed to become established before the sample is applied. Sample is applied to the gel by carefully laying small rectangles of filter paper, impregnated with sample solution, on the gel over a track mark on the template. If necessary, the same sample solution can be applied at different positions, i.e. at different pH values, on the gel. Samples are drawn out of the filter paper and into the gel by electrophoresis and by diffusion. When this has happened the potential is switched off and the sample applicator papers are removed, so that they do not subsequently distort the electrical field.
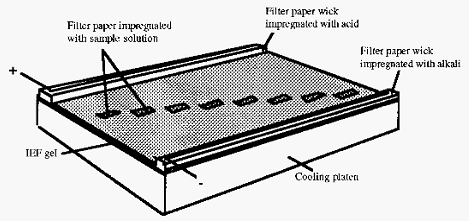
Figure 5. Sketch of apparatus used for analytical flat bed IEF.
After focusing is complete, the gel is removed and stained to reveal the position of the protein bands. Ampholytes are polyamino compounds and at most pH values they will react with and precipitate dyes such as Coomassie blue. To obviate this, the ampholytes can be removed by washing the gel in trichloracetic acid before staining. Asimpler method, however, is to use the principle of the Bradford assay for protein, i.e. to stain with Coomassie blue G-250 in acid solution. Using this approach only the proteins are stained and the ampholytes do not interfere.
In order to determine the pI values of the separated proteins, it is necessary to measure the pH gradient. This can be done, after focusing but before staining, by using a surface-probing pH electrode. However, as with other techniques, once the values for a few proteins have been established, these can be used as standards, and the values of unknowns can be determined by interpolation. Standard proteins, of known pI values can thus be run in parallel with unknowns - on the same gel but in different tracks - and a standard curve of pH vs distance can be constructed from the standards and used to determine the pI values of the unknowns. A table of pI values of standard proteins has been published by Chambers and Rickwood, and pI calibration kits are commercially available.
3.2 Preparative IEF
Preparative IEF21 differs from analytical IEF in that it is done on a much larger scale, i.e. with much more sample and, as the products sought are active protein fractions, provision must be made for recovery of the separated fractions after IEF. The central problem with preparative IEF is stabilization of the system against convection during the focusing process while still being able to elute the separated components at the end of the process, Different approaches to the solution of this problem have been adopted by different authors and by the makers of commercially available apparatus. The most common approach is to conduct the focusing in a convection-stabilized liquid phase. With the proteins and ampholytes being recovered in solution, measurement of the protein concentration and pH of each fraction is relatively straightforward. An alternative approach, suited to smaller-scale preparative uses is to conduct the focusing in a slab gel, to cut out the focused band and electro-elute this. In this case the pH gradient would be measured as described for analytical IEF.
The first approach tried, and commercialized by Pharmacia, was to conduct the focusing in a sucrose gradient, which could be eluted from the apparatus at the end of the experiment. An ingenious, but expensive, apparatus had provisions for cooling the annular focusing column on both its inner and outer surfaces, and valves to isolate the electrode solutions during the elution phase.
An approach used by LKB (which has since merged with Pharmacia) was to use a flat bed of granulated gel. After focusing, the gel bed could be divided into a number of segments, the gel from each segment being scooped out and packed into a mini-column from which the focused protein and ampholytes could be eluted.
In the Bio-Rad Rotoforô apparatus, convection is controlled by conducting the focusing in a horizontal column which is rolled at 1 rpm about its central axis. Rolling serves to negate the effects of gravity and enables the proteins to be focused in free solution. A central ceramic cold finger serves to remove heat. The column is divided into 20 segments by polyester membranes. After focusing, solution in the segments can be rapidly eluted from the side of the column into 20 test-tubes, without mixing. The Rotofor is currently a favoured apparatus for preparative IEF .
Although good results can be obtained by preparative IEF, and for many separations it may be the only practicable method, the technique is constrained by the high initial cost of the apparatus and the cost of the ampholytes consumed in each experiment.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
Margolis, J. and Kenrick, K. G. (1967) Polyacrylamide gel electrophoresis across a molecular sieve gradient. Nature (London) 214, 1334-1336.
Slate, G. G. (1968) Pore-limit electrophoresis on a gradient of polyacrylamide gel. Anal. Biochem. 24, 2 15-2 17.
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















