
HANSCH QSAR ANALYSES
 المؤلف:
James R Hanson
المؤلف:
James R Hanson
 المصدر:
Chemistry and Medicines
المصدر:
Chemistry and Medicines
 الجزء والصفحة:
p37
الجزء والصفحة:
p37
 29-3-2016
29-3-2016
 2070
2070
HANSCH QSAR ANALYSES
A systematic analysis by Hansch of large body of data concerning aromatic compounds led to the establishment of a series of QSAR possessing predictive capability. The work started (1964) by rationalizing the substituent effects on the plant growth promoting properties of phenoxyacetic acids and second, by examining the bacteriocidal action of relatives of chloramphenicol 2.50. It was then extended to many other series of compounds such as the toxic action of substituted phenols on bacteria and the insecticidal action of substituted benzoic acids. Hansch considered the contributions of substituents initially just in terms of their electronic and lipo-hydrophilic contributions. Steric effects were introduced later.
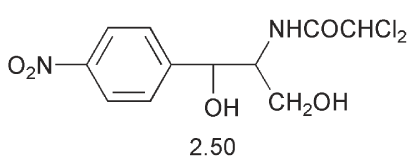
In physical organic chemistry, the Hammett equation related the rate of a reaction for a substituted aromatic compound to the unsubstituted parent in, for example, the hydrolysis of benzoic acid esters, to a combination of a substituent constant (σ) and a reaction constant (ρ).
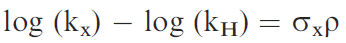
The substituent constant (σ x) can be considered to be a combination of the field and resonance effects. This was used to relate the strengths of aromatic acids and bases.
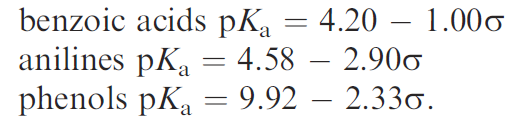
Hansch defined a substituent constant p to reflect the effect of a substituent on the lipo-hydrophilic character of the molecule where πx = log(Px) - log(PH) in which Px and PH are the octanol:water partition coefficients for the substituted and unsubstituted compounds. He then produced equations relating the molar concentration, C, of the compound required to produce the biological effect to p and s which have been expressed in the form: log(l/C) = aπ + ρσ + c where ρ in this case relates to the bioassay.
Although these ideas were initially developed in the context of aromatic substances, the inclusion of steric terms allowed the reasoning to be extended to aliphatic systems. Various measurements of steric terms have been introduced such as the molecular refractivity. It is defined as where n is the refractive index and d is the density of the compound.
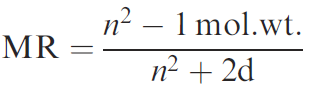
This provides a measure of the polarisibility of a group. Another measure of steric factors is known as the Taft steric parameter (Es) and is based on the effect of a substituent on the rate of acid-catalysed hydrolysis of the substituted methyl acetate.
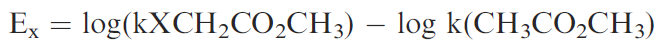
 الاكثر قراءة في الكيمياء الطبية والدوائية
الاكثر قراءة في الكيمياء الطبية والدوائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


