

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Formal Charges
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
p35
26-2-2016
5571
Formal Charges
Closely related to the ideas of bond polarity and dipole moment is the concept of assigning formal charges to specific atoms within a molecule, particularly atoms that have an apparently “abnormal” number of bonds. Look at dimethyl sulfoxide (CH3SOCH3), for instance, a solvent commonly used for preserving biological cell lines at low temperature. The sulfur atom in dimethyl sulfoxide has three bonds rather than the usual two and has a formal positive charge.
The oxygen atom, by contrast, has one bond rather than the usual two and has a formal negative charge. Note that an electrostatic potential map of dimethyl sulfoxide shows the oxygen as negative (red) and the sulfur as relatively positive (blue), in accordance with the formal charges.
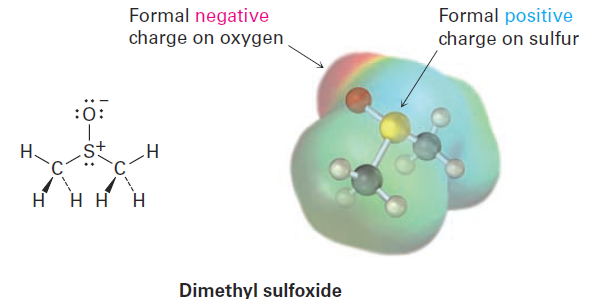
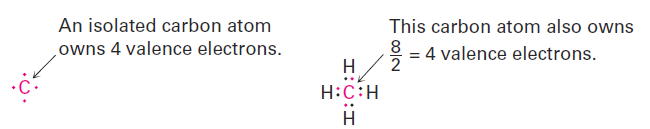
Formal charges, as the name suggests, are a formalism and don’t imply the presence of actual ionic charges in a molecule. Instead, they’re a device for electron “bookkeeping” and can be thought of in the following way: a typical covalent bond is formed when each atom donates one electron. Although the bonding electrons are shared by both atoms, each atom can still be considered to “own” one electron for bookkeeping purposes. In methane, for instance, the carbon atom owns one electron in each of the four C - H bonds. Because a neutral, isolated carbon atom has four valence electrons, and because the carbon atom in methane still owns four, the methane carbon atom is neutral and has no formal charge.
The same is true for the nitrogen atom in ammonia, which has three covalent N- H bonds and two nonbonding electrons (a lone pair). Atomic nitrogen has five valence electrons, and the ammonia nitrogen also has five—one in each of three shared N- H bonds plus two in the lone pair. Thus, the nitrogen atom in ammonia has no formal charge.
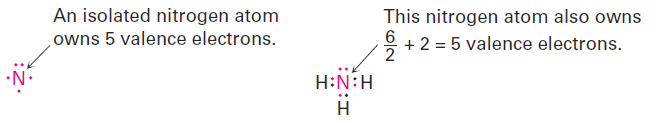
The situation is different in dimethyl sulfoxide. Atomic sulfur has six valence electrons, but the dimethyl sulfoxide sulfur owns only five—one in each of the two S- C single bonds, one in the S- O single bond, and two in a lone pair. Thus, the sulfur atom has formally lost an electron and therefore has a positive charge. A similar calculation for the oxygen atom shows that it has formally gained an electron and has a negative charge. Atomic oxygen has six valence electrons, but the oxygen in dimethyl sulfoxide has seven—one in the O- S bond and two in each of three lone pairs.
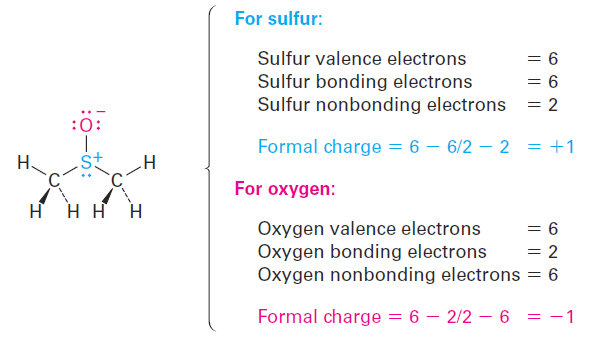
To express the calculations in a general way, the formal charge on an atom is equal to the number of valence electrons in a neutral, isolated atom minus the number of electrons owned by that bonded atom in a molecule. The number of electrons in the bonded atom, in turn, is equal to half the number of bonding electrons plus the nonbonding, lone-pair electrons.
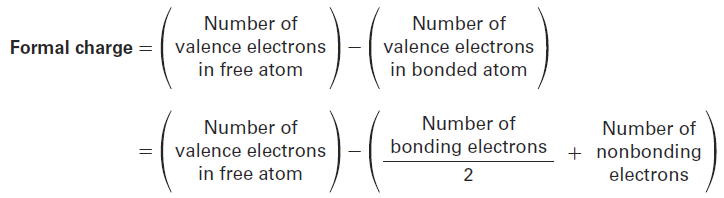
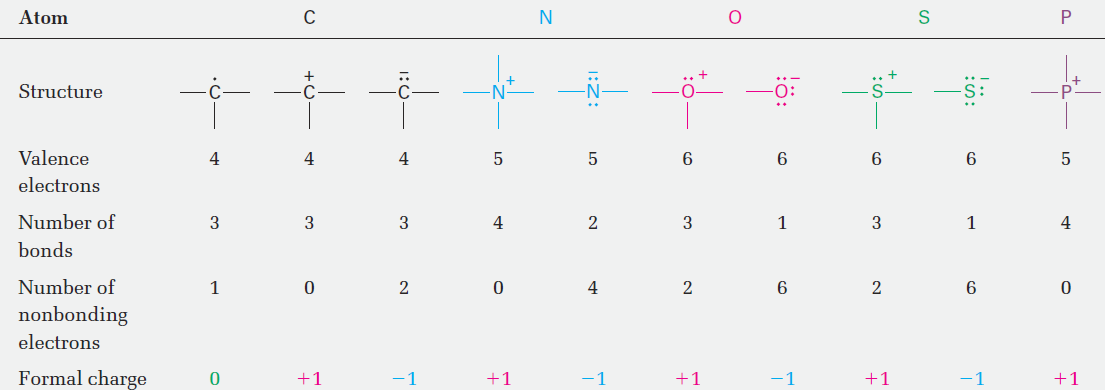
A summary of commonly encountered formal charges and the bonding situations in which they occur is given in Table 1. Although only a bookkeeping device, formal charges often give clues about chemical reactivity, so it’s helpful to be able to identify and calculate them correctly.
Tab le 1 A Summary of Common Formal Charges
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















