

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Polymerization Mechanisms
المؤلف:
Robert O. Ebewele
المصدر:
POLYMER SCIENCE AND TECHNOLOGY
الجزء والصفحة:
p38
25-2-2016
12695
Polymerization Mechanisms
Polymerization processes are classified as step-reaction (condensation) or chain-reaction (addition) polymerization. In this chapter, we will discuss the different types of polymers based on the different polymerization mechanisms.
CHAIN-REACTION POLYMERIZATION
Chain-reaction polymerization, an important industrial method of polymer preparation, involves the addition of unsaturated molecules to a rapidly growing chain. The most common unsaturated compounds that undergo chain-reaction polymerization are olefins, as exemplified by the following reaction of a generalized vinyl monomer.
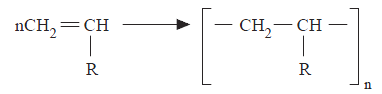
The growing polymer in chain-reaction polymerization is a free radical, and polymerization proceeds via chain mechanism. Chain-reaction polymerization is induced by the addition of free-radical-formingreagents or by ionic initiators. Like all chain reactions, it involves three fundamental steps: initiation, propagation, and termination. In addition, a fourth step called chain transfer may be involved.
A. INITIATION
Initiation involves the acquisition of an active site by the monomer. This may occur spontaneously by the absorption of heat, light (ultraviolet), or high-energy irradiation. But most frequently, initiation of freeradical polymerization is brought about by the addition of small quantities of compounds called initiators.
Typical initiators include peroxides, azo compounds, Lewis acids, and organometallic reagents. However, while initiators trigger initiation of the chain and exert an accelerating influence on polymerization rate, they are not exactly catalysts since they are changed chemically in the course of polymerization. An initiator is usually a weak organic compound that can be decomposed thermally or by irradiation to produce free radicals, which are molecules containing atoms with unpaired electrons. A variety of compounds decompose when heated to form free radicals. Dialkyl peroxides (ROOR), diacylperoxides (RCO–O–O–CO–R), hydroperoxides (ROOH), and azo compounds (RN NR) are typical organic compounds that can be decomposed thermally to produce free radicals. Benzoyl peroxide, azobisisobutyronitrile, and dit.butylperoxide are commonly used free-radical initiators, as illustrated in Equations below.
NR) are typical organic compounds that can be decomposed thermally to produce free radicals. Benzoyl peroxide, azobisisobutyronitrile, and dit.butylperoxide are commonly used free-radical initiators, as illustrated in Equations below.
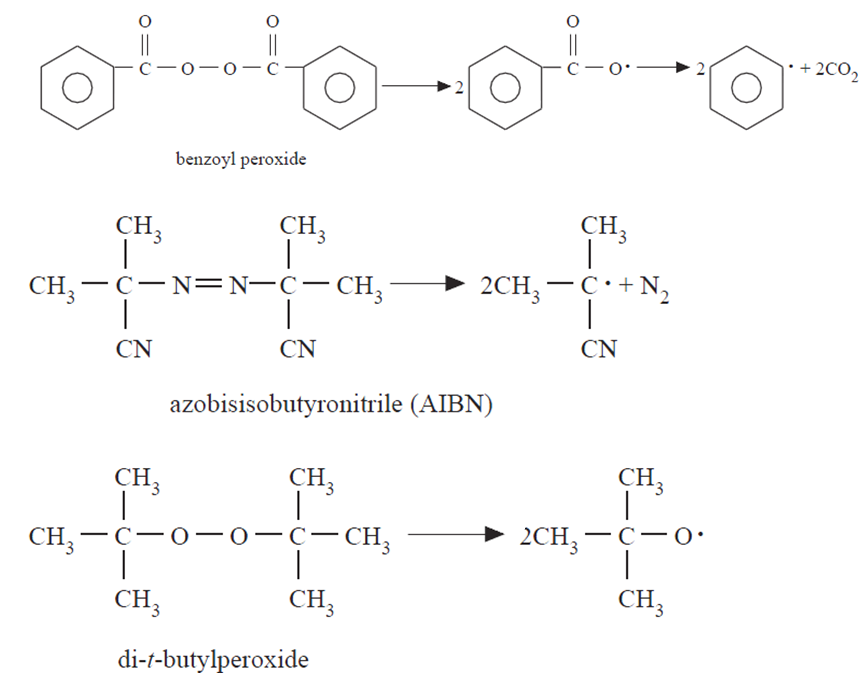
The thermal decomposition of benzoyl peroxide, which takes place between 60 and 90°C, involves the homolytic cleavage of the O–O bond to yield benzoyl free radicals that may react to yield phenyl radicals and carbon dioxide. An example of photochemically induced free-radical formation is the decomposition of azo-bisisobutyronitrile by short-wavelength visible light or near-ultraviolet radiation at temperatures as low as 0 °C, where no thermal initiation occurs.
In free-radical polymerization carried out in aqueous medium, the decomposition of peroxide or persulfate is greatly accelerated by the presence of a reducing system. This method of free-radical initiation is referred to as redox initiation. The initiation resulting from the thermal decomposition of organic compounds discussed above is appropriate only for polymerizations carried out at room temperature or higher. The enhanced rate of free-radical formation in redox reactions permits polymerization at relatively lower temperatures. Typical redox reactions for emulsion polymerization are shown in Equations below.
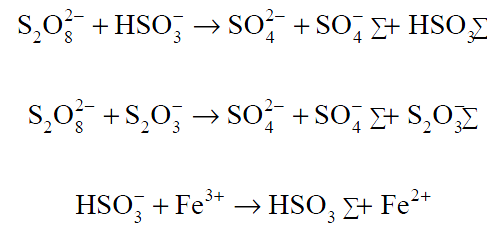
Persulfate ion initiator (e.g., from K2S2O8) reacts with a reducing agent such as a bisulfite ion (e.g., from NaHSO3) to produce radicals for redox initiation (Equations above). Ferric ion may also be used as a source of radicals. Other redox reactions involve the use of alkyl hydroxides and a reducing agent such as ferrous ion (Equation below).

As indicated earlier, free-radical polymerization of some monomers can be initiated by heating or exposing the monomers to light or high-energy irradiation such as X-rays, γ-rays, and α-rays. High-energy irradiation of monomers can be carried out either in bulk or in solution. It is certainly not as selective as photolytic initiation.
When choosing an initiator for free-radical polymerization, the important parameters that must be considered are the temperature range to be used for the polymerization and the reactivity of the radicals formed. The presence of certain promoters and accelerators and the nature of the monomer often affect the rate of decomposition of initiators. For example, the decomposition of benzoyl peroxide may be accelerated at room temperature by employing ternary or quaternary amines. Free-radical initiation processes do not require stringent exclusion of atmospheric moisture, but can be inhibited by substances such as oxygen. Free radicals are inactivated by reaction with oxygen to form peroxides or hydroperoxides.
For monomers such as styrene and methylmethacrylate that are susceptible to such inhibition, initiation reactions are carried out in an oxygen-free atmosphere such as nitrogen. It must be emphasized also that organic peroxides, when subjected to shock or high temperature, can detonate. Therefore these compounds must be handled with caution. The initiation of polymerization occurs in two successive steps. The first step involves the formation of radicals according to the processes discussed above. This may be represented broadly as: 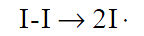
The second step is the addition of the initiator radical to a vinyl monomer molecule:
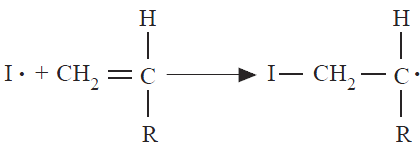
Initiator fragments have been shown by end-group analysis to become part of the growing chain. In commercial practice, 60 to 100% of all the free radicals generated do initiate polymerization.
B. PROPAGATION
During propagation, the initiated monomer described above adds other monomers — usually thousands of monomer molecules — in rapid succession. This involves the addition of a free radical to the double bond of a monomer, with regeneration of another radical. The active center is thus continuously relocated at the end of the growing polymer chain (Equation below).
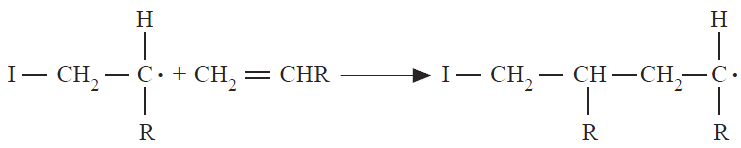
Propagation continues until the growing chain radical is deactivated by chain termination or transfer as discussed below. The substituted carbon atom is regarded as the head and the unsubstituted carbon atom the tail of the vinyl monomer. There are, therefore, three possible ways for the propagation step to occur: headto-tail (Equation above), head-to-head (Equation below), and tail-to-tail (Equation below). A random distribution of these species along the molecular chain might be expected.
It is found, however, that head-totail linkages in which the substituents occur on alternate carbon atoms predominate; only occasional interruptions of this arrangement by head-to-head and tail-to-tail linkages occur. In addition, exclusive head-to-head or tail-to-tail arrangements of monomers in the chain are now known.
TERMINATION
In termination, the growth activity of a polymer chain radical is destroyed by reaction with another free radical in the system to produce polymer molecule(s). Termination can occur by the reaction of the polymer radical with initiator radicals (Equation below). This type of termination process is unproductive and can be controlled by maintaining a low rate for initiation.
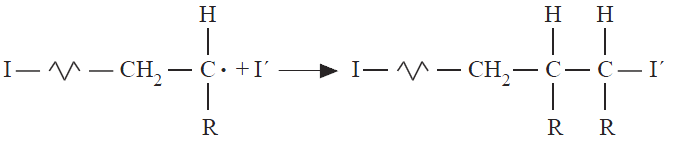
The termination reactions that are more important in polymer production are combination (or coupling) and disproportionation. In termination by combination, two growing polymer chains react with the mutual destruction of growth activity (Equation below), while in disproportionation a labile atom (usually hydrogen) is transferred from one polymer radical to another (Equation above).

Coupling reactions produce a single polymer, while disproportionation results in two polymers from the two reacting polymer chain radicals. The predominant termination reaction depends on the nature of the reacting monomer and the temperature. Since disproportionation requires energy for breaking of chemical bonds, it should become more pronounced at high reaction temperatures; combination of growing polymer radicals predominates at low temperatures.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















