Cis-Trans Isomerism and Conformational Equilibria for Cyclohexane Derivatives
The cis-trans isomerism of cyclohexane derivatives (Section 5-1A) is complicated by conformational isomerism. For example, 4-tert-butylcyclohexyl chloride theoretically could exist in four stereoisomeric chair forms, 1, 2, 3, and 4. trans
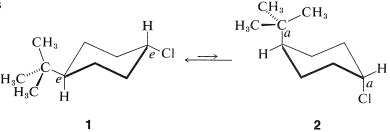
cis
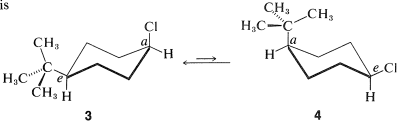
Figure 12-11).
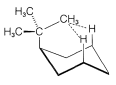
Figure 12-10: 1,3-interactions in a cyclohexane ring with an axial tert-butyl group.
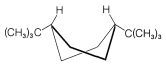
Figure 12-11: Twist-boat conformation of cis-1,4-di-tert-butylcyclohexane.
Use of C13 nmr spectroscopy to determine whether a substituent is in an axial or equatorial position is well illustrated with cis- and trans-4-tert-butylcyclohexanols, 55 and 66:
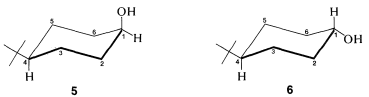
Figure 12-12. At −110o, the equatorial form is 99% of the mixture and is interconverted only very slowly with the 1% of axial form. Despite the strong C13 nmr signals from the equatorial form, the chemical shifts of C3, C5, and CH3 carbons of the axial form are sufficiently different that they can be seen upfield of the methyl resonance of the equatorial form.
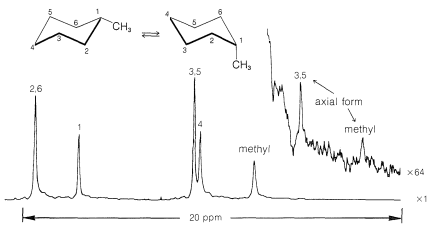
Figure 12-12: Proton-decoupled, 63.1MHz, C13 spectrum of methylcyclohexane at −110o. The upper right curve was taken with the signal sensitivity control turned up by a factor of 64. (Courtesy of Dr. F. A. L. Anet.)
Cyclopentane
The five −CH2− groups of cyclopentane theoretically could form a regular planar pentagon (internal angles of 108o) with only a little bending of the normal C−C−C bond angles. Actually, cyclopentane molecules are not flat. The planar structure has completely eclipsed hydrogens, which makes it less stable by about 10kcal mol−1 than if there were no eclipsed hydrogens. The result is that each molecule assumes a puckered conformation that is the best compromise between distortion of bond angles and eclipsing of hydrogens. The best compromise conformations have the ring twisted with one or two of the −CH2− groups bent substantially out of a plane passed through the other carbons (Figure 12-14). The flexibility of the ring is such that these deformations move rapidly around the ring.
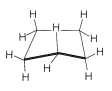
Figure 12-14: Nonplanar conformation of cyclopentane. Notice that the forward carbon is out of the plane of the other four.


