

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Heats of Combustion of Cycloalkanes and Strain Energies
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
............
28-1-2022
2686
Heats of Combustion of Cycloalkanes and Strain Energies
The strain in ring compounds can be evaluated quantitatively by comparing the heats of combustion per CH2 group, as in Table 12-3. The data indicate that cyclohexane is virtually strain-free, because the heat of combustion per CH2 is the same as for alkanes (157.4kcal mol−1). The increase for the smaller rings clearly reflects increasing angle strain and, to some extent, unfavorable interactions between nonbonded atoms. For rings from C7 to C12 there appears to be a residual strain for each additional CH2 of 1 to 1.5kcal mol−1. These rings can be puckered into flexible conformations with normal C−C−C angles, from C7 to C13 such arrangements all have pairs of partially eclipsed or interfering hydrogens. The larger cycloalkanes such as cyclopentadecane appear to be essentially strain-free.
We expect that the total strain in cycloalkanes of the type (CH2)n should decrease rapidly in the order n=2>n=3>n=4. However, the data of Table 12-3 show that the order actually is 3≅4>2. This difference in order often is disguised by dividing the heats of combustion by the numbers of CH2 groups and showing that the heats of combustion per CH2 are at least in the order expected from bond-angle strain. This stratagem does not really solve the problem.
It is important to recognize that when we evaluate strain from the heats of combustion per CH2 group, we are assuming that the C−H bonds have the same strength, independent of nn. However, the bond-dissociation energies of each of the C−H bonds of ethene and cyclopropane are greater than of the C2−H bonds of propane (Table 4-6).
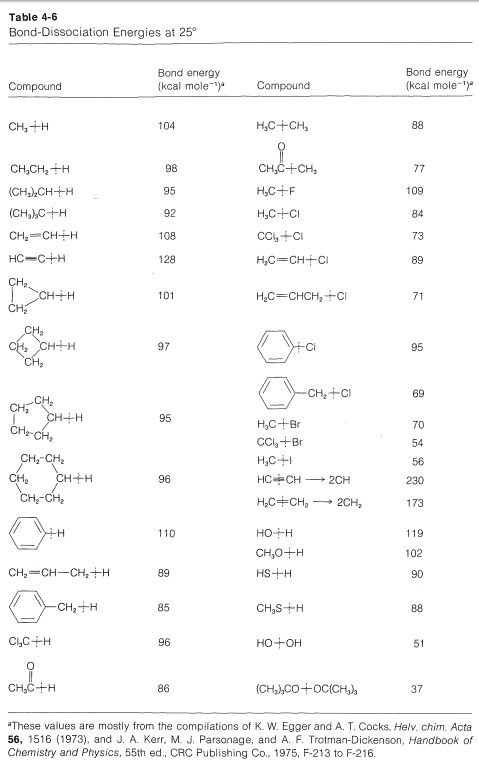
Any amount that these bonds are stronger than normal will make the strain energies judged from heats of combustion appear to be less. If we take the C−H bonds to be on average 2kcal mol−12kcal mol−1 stronger in cyclobutane, 6kcal mol−1 stronger in cyclopropane, and 13kcal mol−113kcal mol−1 in ethene, we can correct the carbon-carbon strain energies accordingly. For cyclobutane the corrected strain then is 8×2 (for the eight C−H bonds) +26.3 (total strain from Table 12-3) =42.3kcal mol−1. The corresponding figures for cyclopropane are 6×6+27.6=63.6kcal mol−1, and for ethene, 4×13+22.4=74.4kcal mol−1. The results support the intuitive expectations by giving larger differences in the right direction for the strain energies of cyclobutane, cyclopropane, and ethene. Whether this analysis is quantitatively correct or not, it does give some indication of why strain energy is not a very precise concept - unless we can reliably estimate the net effects of a strain.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















