

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydration of alkenes
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
14-1-2022
2430
Hydration of alkenes
We mentioned previously that the hydration of alkenes required a strong acid as a catalyst, because water itself is too weak an acid to initiate the proton-transfer step. However, if a small amount of a strong acid such as sulfuric acid is present, hydronium ions, H3O⊕, are formed in sufficient amount to protonate reasonably reactive alkenes, although by no means as effectively as does concentrated sulfuric acid. The carbocation formed then is attacked rapidly by a nucleophilic water molecule to give the alcohol as its conjugate acid, which regenerates hydronium ion by transferring a proton to water. The reaction sequence follows for 2-methylpropene:
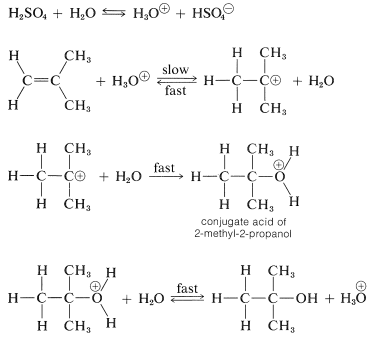
In this sequence, the acid acts as a catalyst because the hydronium ion used in the proton addition step is regenerated in the final step.
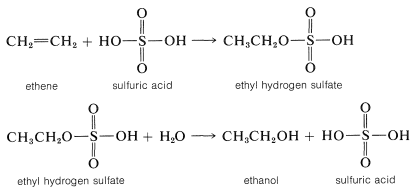
Sulfuric acid (or phosphoric acid) is preferred as an acid catalyst for addition of water to alkenes because the conjugate base, HSO4− (or H2PO4−), is a poor nucleophile and does not interfere in the reaction. However, if the water concentration is kept low by using concentrated acid, addition occurs to give sulfate (or phosphate) esters. The esters formed with sulfuric acid are either alkyl acid sulfates R−OSO3H or dialkyl sulfates (RO)2SO2. In fact, this is one of the major routes used in the commercial production of ethanol and 2-propanol. Ethen and sulfuric acid give ethyl hydrogen sulfate, which reacts readily with water in a second step to give ethanol:
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















