

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Pyrimidine Synthesis and Degradation
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
18-11-2021
2153
Pyrimidine Synthesis and Degradation
Unlike the synthesis of the purine ring, which is constructed on a pre-existing ribose 5-phosphate, the pyrimidine ring is synthesized before being attached to ribose 5-phosphate, which is donated by PRPP. The sources of the atoms in the pyrimidine ring are glutamine, CO2, and aspartate (Fig. 1).
Figure 1: Sources of the individual atoms in the pyrimidine ring. CO2 = carbon dioxide.
A. Carbamoyl phosphate synthesis
The regulated step of this pathway in mammalian cells is the synthesis of carbamoyl phosphate from glutamine and CO2, catalyzed by carbamoyl phosphate synthetase (CPS) II. CPS II is inhibited by uridine triphosphate (the end product of this pathway, which can be converted into the other pyrimidine nucleotides) and is activated by PRPP. [Note: Carbamoyl phosphate, synthesized by CPS I, is also a precursor of urea . Defects in ornithine transcarbamylase of the urea cycle promote pyrimidine synthesis because of increased availability of carbamoyl phosphate. A comparison of the two enzymes is presented in Figure 2.]
Figure 2: Summary of the differences between carbamoyl phosphate synthetase (CPS) I and II. PRPP = 5-phosphoribosyl-1-pyrophosphate; UTP = uridine triphosphate.
B. Orotic acid synthesis
The second step in pyrimidine synthesis is the formation of carbamoylaspartate, catalyzed by aspartate transcarbamoylase. The pyrimidine ring is then closed by dihydroorotase. The resulting dihydroorotate is oxidized to produce orotic acid (orotate), as shown in Figure 3. The human enzyme that produces orotate, dihydroorotate dehydrogenase, is a flavin mononucleotide-containing protein of the inner mitochondrial membrane. All other enzymes in pyrimidine biosynthesis are cytosolic. [Note: The first three enzymic activities in this pathway (CPS II, aspartate transcarbamoylase, and dihydroorotase) are actually three different catalytic domains of a single polypeptide known as CAD from the first letter in the name of each domain. This is an example of a multifunctional or multicatalytic polypeptide that facilitates the ordered synthesis of an important compound. Synthesis of the purine nucleotide IMP also involves multifunctional proteins.]
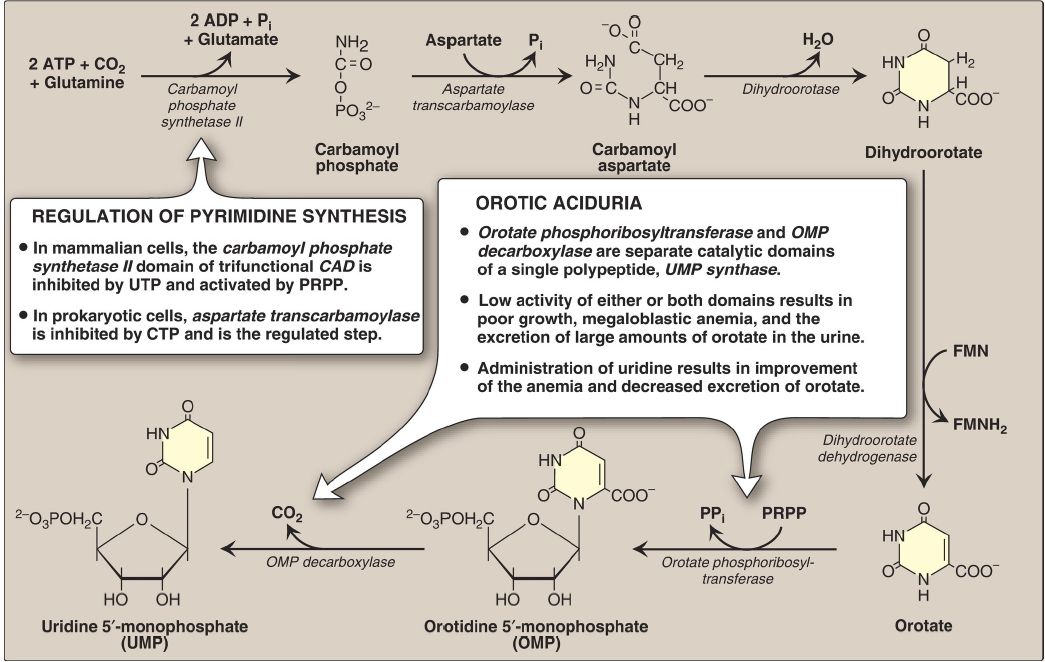
Figure3: De novo pyrimidine synthesis. ADP = adenosine diphosphate; Pi = inorganic phosphate; FMN(H2) = flavin mononucleotide; CTP = cytidine triphosphate; PRPP = 5-phosphoribosyl-1-pyrophosphate; PPi = pyrophosphate.
C. Pyrimidine nucleotide synthesis
The completed pyrimidine ring is converted to the nucleotide orotidine monophosphate (OMP) in the second stage of pyrimidine nucleotide synthesis (see Fig. 3). As seen with the purines, PRPP is the ribose 5-phosphate donor. The enzyme orotate phosphoribosyltransferase produces OMP and releases pyrophosphate, thereby making the reaction biologically irreversible. [Note: Both purine and pyrimidine synthesis require glutamine, aspartic acid, and PRPP as essential precursors.] OMP (orotidylate) is decarboxylated to uridine monophosphate (UMP) by orotidylate decarboxylase. The phosphoribosyltransferase and decarboxylase activities are separate catalytic domains of a single polypeptide called UMP synthase. Hereditary orotic aciduria (a very rare disorder) may be caused by a deficiency of one or both activities of this bifunctional enzyme, resulting in orotic acid in the urine (see Fig. 3). UMP is sequentially phosphorylated to UDP and UTP. [Note: The UDP is a substrate for
ribonucleotide reductase, which generates dUDP. The dUDP is phosphorylated to dUTP, which is rapidly hydrolyzed to dUMP by UTP diphosphatase (dUTPase). Thus, dUTPase plays an important role in reducing availability of dUTP as a substrate for DNA synthesis, thereby preventing erroneous incorporation of uracil into DNA.]
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















