

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Overview of Amino Acids
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
2373
Overview of Amino Acids
Proteins are the most abundant and functionally diverse molecules in living systems. Virtually every life process depends on this class of macromolecules. For example, enzymes and polypeptide hormones direct and regulate metabolism in the body, whereas contractile proteins in muscle permit movement. In bone, the protein collagen forms a framework for the deposition of calcium phosphate crystals, acting like the steel cables in reinforced concrete. In the bloodstream, proteins, such as hemoglobin and albumin, transport molecules essential to life, whereas immunoglobulins fight infectious bacteria and viruses. In short, proteins display an incredible diversity of functions, yet all share the common structural feature of being linear polymers of amino acids.
STRUCTURE
Although >300 different amino acids have been described in nature, only 20 are commonly found as constituents of mammalian proteins. [Note: These standard amino acids are the only amino acids that are encoded by DNA, the genetic material in the cell . Nonstandard amino acids are produced by chemical modification of standard amino acids . Each amino acid has a carboxyl group, a primary amino group (except for proline, which has a secondary amino group), and a distinctive side chain (R group) bonded to the α-carbon atom. At physiologic pH (~7.4), the carboxyl group is dissociated, forming the negatively charged carboxylate ion (−COO−), and the amino group is protonated (−NH3+) (Fig. 1.1A). In proteins, almost all of these carboxyl and amino groups are combined through peptide linkage and, in general, are not available for chemical reaction except for hydrogen bond formation (Fig. 1.1B).
Thus, it is the nature of the side chains that ultimately dictates the role an amino acid plays in a protein. Therefore, it is useful to classify the amino acids according to the properties of their side chains, that is, whether they are nonpolar (have an even distribution of electrons) or polar (have an uneven distribution of electrons, such as acids and bases) as shown in Figures 1.2 and 1.3.

Figure 1.1 A, B. Structural features of amino acids.

Figure 1.2 Classification of the 20 standard amino acids, according to the charge and polarity of their side chains at acidic pH, is shown here and continues in Figure 1.3. Each amino acid is shown in its fully protonated form, with dissociable hydrogen ions represented in red. The pK values for the α-carboxyl and α-amino groups of the nonpolar amino acids are similar to those shown for glycine.
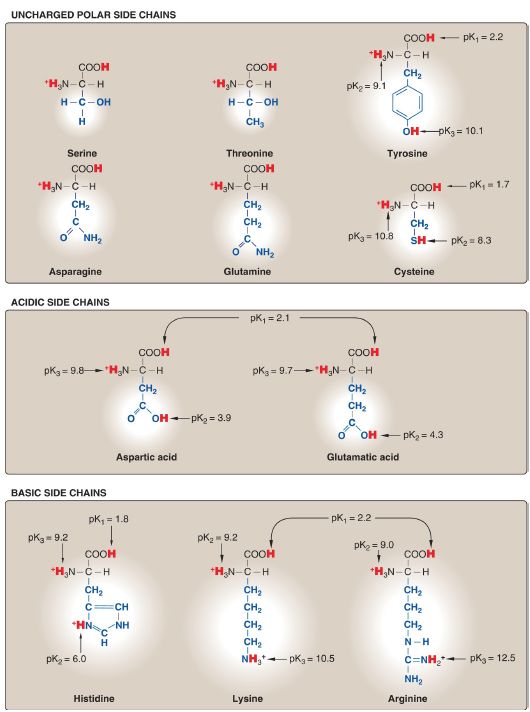
Figure 1.3 Classification of the 20 standard amino acids, according to the charge and polarity of their side chains at acidic pH (continued from Fig. 1.2). [Note: At physiologic pH (7.35 to 7.45), the α-carboxyl groups, the acidic side chains, and the side chain of free histidine are deprotonated.]
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















