

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Transposons Form Superfamilies and Families
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
23-4-2021
3209
Transposons Form Superfamilies and Families
KEY CONCEPTS
- Superfamilies of transposons are defined by the sequence of the transposase.
- Transposon families have both autonomous and nonautonomous members.
- Autonomous transposons code for proteins that enable them to transpose.
- Nonautonomous transposons cannot catalyze transposition, but they can transpose when an autonomous element provides the necessary proteins.
- Autonomous transposons have changes of phase, when their properties alter in association with changes in the state of methylation.
Most eukaryotic genomes contain multiple superfamilies of DNAbased (class II) transposons. Transposon superfamilies are defined by the sequences of their encoded transposases. Transposons may occupy a significant part of the genome; for example, the maize genome has roughly doubled in overall size in the last 6 million years due to transposon activity, and transposons occupy 25% of the genome of the frog Xenopus tropicalis. In humans, only 3% of the genome is composed of DNA-based transposons (our genome contains many more class I elements), but the 3% represents nearly 400,000 individual transposable elements. The members of transposon families can be divided into two classes:
- Autonomous transposons have the ability to excise and transpose. As a result of the continuing activity of an autonomous transposon, its insertion at any locus creates an unstable, or “mutable,” allele. Loss of the autonomous transposon itself, or of its ability to transpose, converts a mutable allele to a stable allele.
- Nonautonomous transposons are stable; they do not transpose or suffer other spontaneous changes in condition. They become unstable only when an autonomous member of the same family is present elsewhere in the genome. When complemented in trans by an autonomous element, a nonautonomous element displays the usual range of activities associated with autonomous elements, including the ability to transpose to new sites. Nonautonomous transposons are derived from autonomous transposons by loss of trans-acting functions needed for transposition.
Within the superfamilies, families of transposons consist of a single type of autonomous element accompanied by a variety of nonautonomous elements. A nonautonomous element is placed in a family by its ability to be activated in trans by the autonomous elements. The relationship between active transposons and nonautonomous partners is depicted in FIGURE 1. Different plant and animal species have differing numbers of active transposons, but in general only a limited number of transposons, if any, are known to be active in a given species. Very few endogenous DNA-based transposons are currently active in vertebrates, whereas plants harbor a large number of active elements.
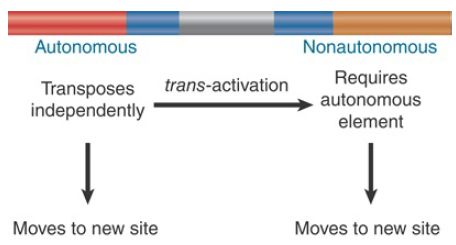
FIGURE 1. Each transposon family has both autonomous and nonautonomous members. Autonomous elements are capable of transposition. Nonautonomous elements are deficient in transposition.
Transposon superfamilies also have differing distributions in nature. Some are highly species restrictive, whereas others are able to move between quite distantly related hosts. For example, P elements (see the section in this chapter titled The Role of Transposable Elements in Hybrid Dysgenesis) are restricted to the Drosophila genus, whereas transposons in the Tc1/mariner superfamily (originally identified in Caenorhabditis elegans and Drosophila mauritiana) are remarkably widespread and have been identified in fungi, ciliates, plants, and animals. These promiscuous elements have been adapted for use as transgene vectors in vertebrates (most notably the versatile Sleeping Beauty element), and seem able to function in nearly any species due to their lack of dependence on specific host factors for transposition. One of the only autonomous DNA transposons known in vertebrates, Tol1 (a member of the hAT superfamily discovered in medaka fish), also appears to be active when transferred to other species, including mammals.
Characterized at the molecular level, most transposons share the usual form of organization—inverted repeats at the ends and short direct repeats in the adjacent target DNA—but otherwise vary in size and coding capacity. All families of transposons share the same type of relationship between the autonomous and nonautonomous elements. The autonomous elements have open reading frames between the terminal repeats, whereas the nonautonomous elements do not code for functional proteins.
Sometimes the internal sequences are related to those of autonomous elements; at other times they are composed of fragments of genes that have been captured between transposoninverted repeats. Some examples of transposon families are described in the paragraphs that follow.
The first transposons were originally identified in maize, which contains a number of active transposons. The Mutator transposon is the most active and mutagenic of all maize transposons. The autonomous element MuDR contains the genes mudrA (which encodes the MURA transposase) and mudrB (which encodes MURB, an accessory protein required for integration). The ends of the elements are marked by 200-bp inverted repeats.
Nonautonomous Mutator elements—basically any units that have the inverted repeats, but that may not have any internal sequence relationship to MuDR—are also mobilized by MURA and MURB.
Mutator elements in maize are the founding members of the MULE (Mu-like element) superfamily of transposons, which are present in bacteria, fungi, plants, and animals. The prototypical transposons, also originally found in maize, are members of the Ac/Ds family, first discovered by Barbara McClintock in the 1940s (and for which she received the Nobel Prize in 1983). FIGURE 2 summarizes their structures. Their molecular characteristics are described further here to illustrate some of the typical relationships between autonomous and nonautonomous family members. Although this example is from maize, the principles apply to transposon families in any species.
Most of the length of the autonomous Ac (Activator) element is occupied by a single gene consisting of five exons. The product is the transposase. The element itself ends in inverted repeats of 11 bp, and a target sequence of 8 bp is duplicated at the site of insertion.
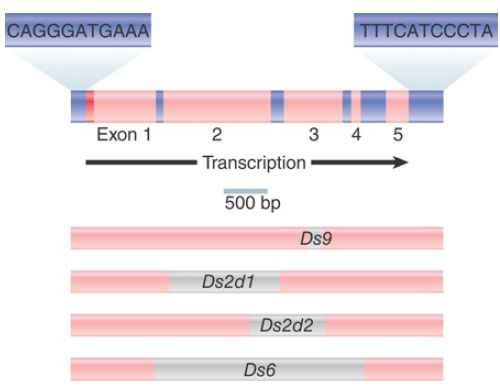
FIGURE 2. The Ac element has five exons (pink) that encode a transposase; Ds elements have internal deletions (gray).
Ds (Dissociator) elements vary in both length and sequence, but are related to Ac. They end in the same 11-bp inverted repeats. They are shorter than Ac, and the length of deletion varies. At one extreme, the element Ds9 has a deletion of only 194 bp. In a more extensive deletion, the Ds6 element retains a length of only 2 kb, representing 1 kb from each end of Ac. A complex double Ds element has one Ds6 sequence inserted in reverse orientation into another.
Nonautonomous elements lack internal sequences but possess the terminal inverted repeats (and possibly other sequence features). Some nonautonomous elements are derived from autonomous elements by deletions (or other changes) that inactivate the transacting transposase but leave the sites (including the termini) on which the transposase acts intact. Their structures range from minor (but inactivating) mutations of Ac to sequences that have major deletions or rearrangements. At another extreme, the Ds1 family members comprise short sequences whose only relationship to Ac lies in the possession of terminal inverted repeats. Elements of this class need not be directly derived from Ac, but could be derived by any event that generates the inverted repeats. Their existence suggests that the transposase recognizes only the terminal inverted repeats or possibly the terminal repeats in conjunction with some short internal sequence.
Ds1 elements are just one example of a widespread form of DNAtype elements called MITEs (miniature inverted repeat transposable elements). These are very short derivatives of autonomous elements found in many eukaryotes that can be present in tens or hundreds of thousands of copies in a given genome. They range from 300 to 500 bp, and generate 2- to 3-bp target site duplications. Unlike many other classes of transposons in plants, MITEs are often found in or near genes.
Transposition of Ac/Ds occurs by a nonreplicative cut-and-paste mechanism that involves double-stranded breaks followed by integration of the released element. The mechanism of transposition is similar to that described for Tn5 and Tn10 . It is accompanied by its disappearance from the donor location. Transposition of Ac/Ds almost always occurs soon after the donor element has been replicated. These features resemble transposition of the bacterial element Tn10. The cause is the same: Transposition does not occur when the DNA of the transposon is methylated on both strands (the typical state before replication); it is activated when the DNA is hemimethylated (the typical state immediately after replication). The recipient site is frequently on the same chromosome as the donor site, and often is quite close to it. Note that if transposition is from a replicated region of a chromosome into an unreplicated region, the transposition event will result in a net increase in the copy number of the element; one chromatid will carry a single copy of the transposon, and the second chromatid will carry two copies. This ensures that elements such as Ac can increase their copy number, even though transposition is not duplicative.
Replication generates two copies of a potential Ac/Ds donor, but usually only one copy actually transposes. What happens to the donor site? The rearrangements that are found at sites from which controlling elements have been lost can be explained in terms of the consequences of a chromosome break. Based on the sequence of the donor site following excision, the majority of the breaks caused by Ac excision appear to be repaired using nonhomologous end joining, which usually creates sequence alterations, or “transposon footprints,” at the excision sites. If the resulting transposon footprint restores functionality to the gene in which the Ac element had been inserted, the result is a reversion event. Otherwise, the result is a stable, nonfunctional gene. In contrast, the mode of Mu element transposition appears to vary depending on the tissue type. Late during somatic development, transposition is similar to that observed for Ac. In germinal tissues, though, the vast majority of transposition events are effectively replicative, perhaps due to gap repair using the sister chromatid as a template.
Autonomous and nonautonomous elements are subject to a variety of changes in their condition. Some of these changes are genetic; others are epigenetic. The major change is (of course) the conversion of an autonomous element into a nonautonomous element, but further changes may occur in the nonautonomous element. cis-acting defects may render a nonautonomous element impervious to autonomous elements. Thus, a nonautonomous element may become permanently stable because it can no longer be activated to transpose.
Autonomous elements are subject to “changes of phase,” which are heritable (but often unstable) alterations in their properties. These may take the form of a reversible inactivation in which the element cycles between an active and inactive condition during plant development, or they may result in stably inactive elements.
Phase changes in both the Ac and Mu types of autonomous element are associated with changes in the methylation of DNA. The inactive forms of all elements are methylated at cytosine residues. In most cases, it is not known what triggers this loss of activity, but in the case of MuDR epigenetic silencing can be triggered by a derivative of MuDR that is duplicated and inverted relative to itself. This rearrangement results in the production of a hairpin RNA, in which two parts of the transcript are perfect complements to each other. The resulting double-stranded RNA is processed by cellular factors into small RNAs that, in turn, trigger methylation and transcriptional gene silencing of the MuDR element .
The effect of methylation is common generally among transposons in plants and other organisms that methylate their DNA. The best demonstration of the effect of methylation on activity comes from observations made with the Arabidopsis mutant ddm1, which causes a genome-wide loss of methylation. Among the targets that lose methyl groups is a family of transposons related to MuDR.
Direct analysis of genome sequences shows that the demethylation and associated modification of histone tails allow transposition events to occur. Methylation is probably the major mechanism that is used to prevent transposons from damaging the genome by transposing too frequently. Transposons appear to be targeted for methylation because they are far more likely to produce doublestranded or otherwise aberrant transcripts that can be used to guide sequence-specific DNA methylation using small RNA produced from those transcripts. In addition, a class of small RNAs expressed in germ cells is enriched in transposable elements and other repetitive sequences, and their expression results in transposon repression. The first RNAs described in this class are the piwi-interacting RNAs of Drosophila and are proposed to protect the germline against sterilizing transposition events; homologs in mice appear to play the same role during spermatogenesis. Once methylation of a transposon has been established, it can be heritably maintained over many generations. In plants and animals that methylate their DNA, the vast majority of transposons are epigenetically silenced in this way.
Transposition may be self-regulating, analogous to the immunity effects displayed by bacterial transposons. An increase in the number of Ac elements in the genome decreases the frequency of transposition. The Ac element may code for a repressor of transposition; the activity could be carried by the same protein that provides transposase function. Additionally, derivatives of some transposons, such as those of P elements in Drosophila, encode truncated proteins that can repress the activity of autonomous elements in somatic tissue .
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















