

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
min and noc/slm Genes Regulate the Location of the Septum
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
30-3-2021
3060
min and noc/slm Genes Regulate the Location of the Septum
KEY CONCEPTS
-The location of the septum is controlled by minC, -D, and -E, and by noc/slmA.
-The number and location of septa are determined by the ratio of MinE/MinCD.
-Dynamic movement of the Min proteins in the cell sets up a pattern in which inhibition of Z-ring assembly is highest at the poles and lowest at mid-cell.
-SlmA/Noc proteins prevent septation from occurring in the space occupied by the bacterial chromosome.
Clues to the localization of the septum were first provided by minicell mutants. The original minicell mutation lies in the locus minB. Deletion of minB generates minicells by allowing septation to occur near the poles instead of at mid-cell, and therefore the role of the wild-type minB locus is to suppress septation at the poles.
The minB locus consists of three genes, minC, -D, and -E. The products of minC and minD form a division inhibitor. MinD is required to activate MinC, which prevents FtsZ from polymerizing into the Z-ring.
Expression of MinCD in the absence of MinE, or overexpression even in the presence of MinE, causes a generalized inhibition of division. The resulting cells grow as long filaments without septa.
Expression of MinE at levels comparable to MinCD confines the inhibition to the polar regions, thus restoring normal growth. The determinant of septation at the proper (mid-cell) site is, therefore, the ratio of MinCD to MinE.
The localization activities of the Min system are due to a remarkable dynamic behavior of MinD and MinE, which is illustrated in FIGURE 1. MinD, an ATPase, oscillates from one end of the cell to the other on a rapid time scale. MinD-ATP binds to and accumulates at the bacterial lipid membrane at one pole of the cell, is released, and then rebinds to the opposite pole. The periodicity of this process takes about 30 seconds, so that multiple oscillations occur within one bacterial cell generation. MinC, which cannot move on its own, oscillates as a passenger protein bound to MinD. MinE forms a ring around the cell at the edge of the zone of MinD. The MinE ring moves toward MinD at the poles and is necessary for ATP hydrolysis and the release of MinD from the membrane. The MinE ring then disassembles and reforms at the edge of the MinD zone that forms at the opposite pole. MinD and MinE are each required for the dynamics of the other. The consequence of this dynamic behavior is that the concentration of the MinC inhibitor is lowest at mid-cell and highest at the poles, which directs FtsZ assembly at mid-cell and inhibits its assembly at the poles.
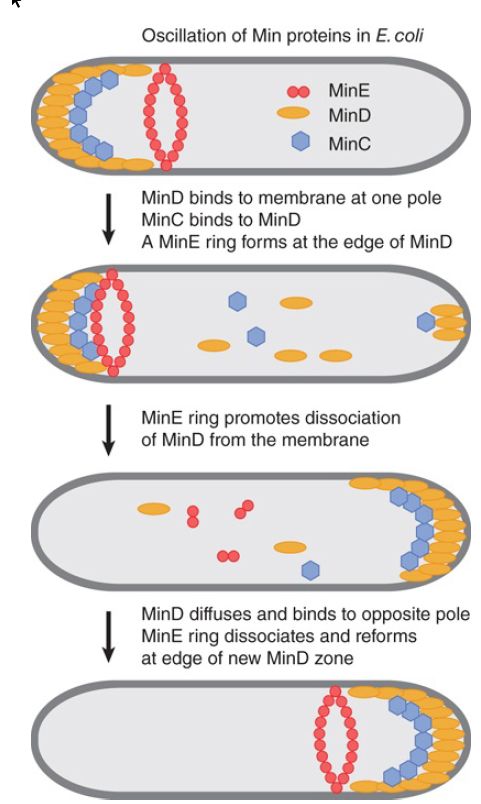
FIGURE 9.8 MinCD is a division inhibitor whose action is confined to the polar sites by MinE.
Another process, called nucleoid occlusion, prevents Z-ring formation over the bacterial chromosome and thus prevents the septum from bisecting an individual chromosome at cell division. A protein called SlmA, which interacts with FtsZ, is necessary for nucleoid occlusion in E. coli. SlmA binds specifically to at least 24 sites on the bacterial chromosome. DNA binding activates SlmA to antagonize the polymerization of FtsZ, which prevents septum formation in this region of the cell. In Bacillus subtilis, a DNAbinding protein called Noc performs a similar nucleoid occlusion role, but by a different mechanism. Noc interacts directly with the membrane, rather than with FtsZ, and this interaction interferes with the assembly of the cell division machinery. The bacterial nucleoid takes up a large volume of the cell, and as a result this process restricts Z-ring assembly to the limited nucleoid-free spaces at the poles and mid-cell. The combination of nucleoid occlusion and the Min system promotes the Z-rings to form, and thus cell division to occur, at mid-cell.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















