

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Theory of biosensor
المؤلف:
John M Walker and Ralph Rapley
المصدر:
Molecular Biology and Biotechnology 5th Edition
الجزء والصفحة:
20-1-2021
2008
Theory of biosensor
In the absence of diffusion effects (see later), most biological reactions and binding processes can be described in terms of saturation kinetics:
biological material + analyte → bound analyte (1)
The bound analyte is then detected optically or by weight or gives rise to a biological action, so generating the electronic response. This electronic or optical response varies with the extent of binding or biological response, which, in turn, varies with the concentration of the bound analyte. Apart from the logarithmic relationship in potentiometric biosensors, the optical, biological and electronic responses are often proportional:
bound analyte → biological or optical response→ electronic response →electronic response

where the half-saturation constant is equal to the analyte concentration which gives rise to half the maximum electronic response possible (Figure ). The response is linear, to within 95%, at analyte concentrations up to about one-twentieth of the half-saturation constant A biosensor may be used over a wider, non-linear range if it has compensatory electronics. This relationship holds for both reacting (e.g. enzymes) and nonreacting (e.g. immunosorption) processes.
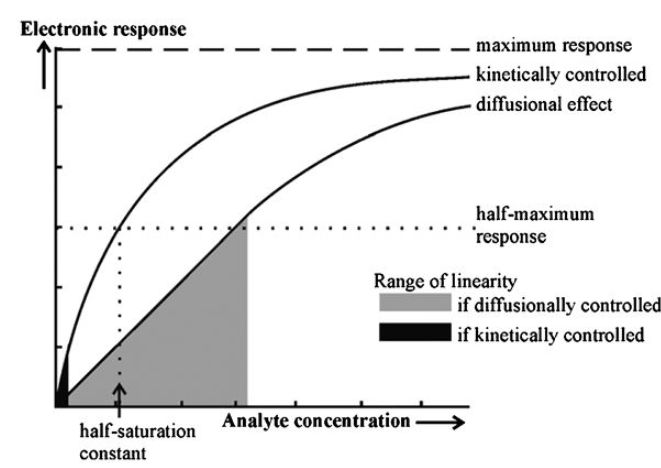
Figure . The range of response of a biosensor under biocatalytic kinetic and diffusional control.
Where the analyte reacts as part of the biological response [e.g. during a biocatalytic reaction utilising enzyme(s) and/or microbial cells], an additional factor is the diffusion of the analyte from the bulk of the solution to the reactive surface. If this rate of diffusion is less than the rate at which the analyte would otherwise react, there follows a reduction in the local concentration of analyte undergoing reaction. The rate of diffusion increases as the concentration gradient increases:

where the analyte concentration gradient is given by the difference between the bulk analyte concentration and the local (microenvironmental) analyte concentration on the sensing surface of the biosensor divided by the distance through which the analyte must diffuse.
As most biocatalytic biosensor configurations utilise a membraneentrapped biocatalyst, this concentration gradient depends not only on the analyte concentration in the bulk and within the membrane but also on the membrane’s thickness. The thicker the membrane, the greater is the diffusive distance from the bulk of the solution to the distal sensing surface of the biosensor and the greater is the amount of biocatalyst encountered. Both effects increase the likelihood that the overall reaction will be controlled by diffusion. Hence such biosensors can be designed to be under diffusional or kinetic control by varying the membrane thickness. When the rate of analyte diffusion is slower than the rate at which the biocatalyst can react, the electronic response decreases due to the lower level of analyte available for reaction. A steady state is rapidly established when the rate of arrival of the analyte equals its rate of reaction. This steady-state condition may be determined wholly by the rate of diffusion (diffusional control) or wholly by the rate of reaction (kinetic control) or by an intermediate dependence. Where the reaction rate depends solely on the rate of diffusive flux of the analyte, this determines the electronic response:
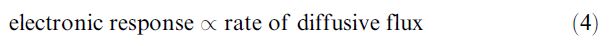
As the rate of diffusion depends on the bulk concentration of the analyte, this electronic response is linearly related to the bulk analyte concentration and, most importantly and intriguingly, is independent of the properties of the enzyme. Thus, the biosensor is linear over a much wider range of substrate concentrations (see Figure ) and relatively independent of changes in the pH and temperature of the biocatalytic membrane, provided that the system remains diffusion controlled. It should be noted, however, that under these conditions the response is reduced relative to a system containing the same amount of biocatalyst but not diffusionally limited. Maximum sensitivity to analyte concentration would be accomplished by the utilisation of thin membranes containing a high biocatalyst activity and a well-stirred analyte solution.
The overall kinetics of most biosensor configurations are difficult to predict. They depend on the diffusivities in the bulk phase and within the biocatalytic volume, the nature, porosity and physical properties of any membrane, the intrinsic biocatalytic kinetics, the electronic transduction process and kinetics, the way in which the analyte is presented and on other non-specific factors. Generally, such overall kinetics are determined experimentally using the complete biosensor and, hence, it is very important that the biosensor configuration is reproducible.
In biosensors utilising binding only, such as immunosensors, the major problem encountered is non-specific absorption that blocks the binding sites. There is need to minimise this and maximise the specific binding. As binding is an equilibrium process, high sensitivity necessitates a very high affinity between the analyte and the sensor surface
 الاكثر قراءة في مواضيع عامة في التقانة الإحيائية
الاكثر قراءة في مواضيع عامة في التقانة الإحيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















