

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Properties of Immobilised Biocatalysts
المؤلف:
John M Walker and Ralph Rapley
المصدر:
Molecular Biology and Biotechnology 5th Edition
الجزء والصفحة:
9-1-2021
2618
Properties of Immobilised Biocatalysts
When a biocatalyst is immobilised, its fundamental characteristics are usually changed in one way or another and the change may be a drawback or an improvement. The nature of the alteration depends on the inherent properties of the biocatalyst and additional characteristics imposed by the support material on the biocatalyst, substrate and product. It is very difficult to quantify these properties and characteristics given the diversity of biocatalysts, support materials and methods of immobilisation. Consequently, it has proved impossible to predict completely what effect a particular immobilisation will have on an enzyme or cell or on the reaction that it catalyses, and the only recourse is to evaluate a number of methods to discover and develop a system that provides the greatest positive improvement for the application under consideration.
1. Stability
The two most important properties that may be changed by immobilisation are stability and catalytic activity. Stability is defined as an ability to resist alteration and in the context of biocatalyst stability it is important to distinguish several different types of stability:
- inactivation by heat;
- disruption by chemicals;
- digestion by proteases or cells;
- inactivation by change in pH;
- loss of catalytic activity during storage;
- loss of catalytic activity due to process operations.
The various types are not necessarily interdependent and an observed increase in heat stability does not indicate that there will be a corresponding increase in storage stability or operational stability. Although immobilisation does not guarantee an improvement in stability, it is generally recognised that it does represent a strategy that can be used as a means of developing more stable enzyme preparations.
Generally, it is found that covalent immobilisation is more effective than the other methods at improving enzyme resistance to heat, chemical disruption and pH changes. Disruptants normally induce loss of catalytic activity by causing a considerable alteration in the protein structure of an enzyme . In particular, disruptants disperse the many non-covalent bonds responsible for holding the enzyme polypeptide chain in its highly specific shape or conformation, thus causing the polypeptide chain to unfold with a consequent loss of active site structure and catalytic activity. Given that unfolding is associated with loss of activity, it is probable that multi-point attachment of an enzyme polypeptide chain to a support material provides extra rigidity to the folded protein chain and therefore greater resistance to protein unfolding .
2. Catalytic Activity
Immobilisation almost invariably changes the catalytic activity of an enzyme, and this is clearly reflected in alterations in the characteristic kinetic constants of the enzyme-catalysed reaction. In particular, the maximum reaction velocity (Vmax) obtained with an immobilised
enzyme is usually lower than that obtained with the corresponding soluble enzyme under the same reaction conditions. The Michaelis constant (Km), which reflects the affinity that the enzyme has for its substrate, is usually changed upon immobilisation, indicating that binding of substrate to the active site has been altered. Four principal factors influence the catalytic activity of immobilised enzymes: (a) conformation, (b) steric, (c) micro-environment and (d) diffusion.
The conformation of an enzyme refers to the particular shape adopted by the polypeptide chain, which is essential for maintaining the active site structure . Immobilisation procedures that involve modification or interaction with amino acid residues on the polypeptide chain can sometimes disturb protein structure and thereby affect the enzyme activity. Covalent immobilisation is most likely to cause an alteration in the protein conformation of an enzyme. A steric problem arises if the enzyme is immobilised in a position that causes the active site to be less accessible to the substrate molecules. In solution, a free enzyme molecule is surrounded by a homogeneous micro-environment in which the enzyme is fully integrated with all components of the solution.
Immobilisation creates a heterogeneous micro-environment consisting of two phases, i.e. the immobilised enzyme and the bulk of the solution from which the immobilised enzyme is separated . Therefore, all components of the reaction, substrate, products, activators, ions, etc., are partitioned between the immobilised enzyme phase and the bulk solution phase. This feature can significantly alter the characteristics of an enzyme reaction even if the enzyme molecule itself is not changed by immobilisation. The support material may influence the partitioning effect. If the support material attracts substrate then this can improve catalytic activity. Reaction rate is also reduced by diffusion restriction. As the substrate is consumed, more substrate must diffuse into the enzyme from the bulk solution and product must diffuse away from the active site. This is normally a problem for all forms of immobilised enzymes, but particularly so for encapsulated enzymes .
Diffusional limitations may be divided into two types, external diffusion restriction and internal diffusion restriction. The external type refers to a zone or barrier that surrounds the support material, called the Nernst layer. Substrate molecules can diffuse into this layer by normal convection and by a passive molecular diffusion. If substrate molecules pass through this layer slowly, then this may limit the rate of enzyme reaction. External diffusion restriction can be improved by speeding up the flow of solvent over and through the immobilised enzyme by increasing the stirring rate.
Internal diffusion restrictions are due to a diffusion limitation inside the immobilised enzyme preparation . In this case, diffusion of substrate molecules occurs by a passive molecular mechanism only, which may be more difficult to overcome if it is a seriously limiting factor. The overall rate of diffusion is markedly influenced by the method of immobilisation. Covalent and adsorption procedures cause less diffusion limitation than do entrapment and encapsulation
procedures.
3 .Coenzyme Regeneration
Around 25% of enzymes are oxidoreductases and they catalyse many reactions that are of great interest to biotechnologists.
However, most of these enzymes require participation of a coenzyme such as NAD, NADH, NADP, NADPH or ATP. Coenzymes are chemically changed during reaction and so in effect become an expensive consumable. Hence efficient regeneration of coenzyme is essential
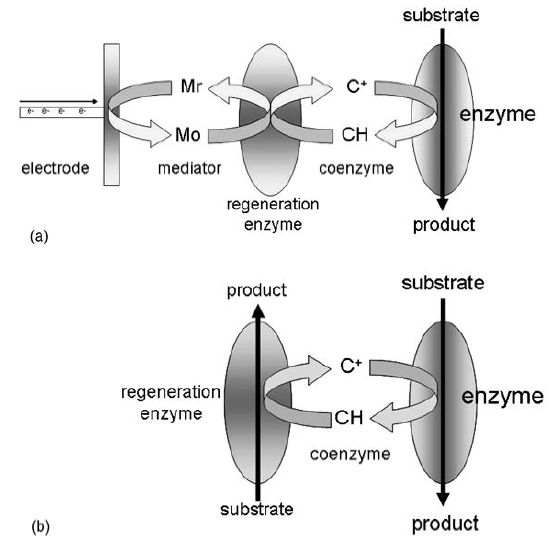
Figure 1. Diagram depicting coenzyme regeneration using (a) an electrode, regeneration enzyme and chemical mediator (Mr, reduced; Mo, oxidised) to recycle the coenzyme and (b) a regeneration enzyme in a reaction that utilises a secondary substrate.
for applications that utilise these enzymes. There are basically two mechanisms to regenerate coenzyme, as depicted in Figure 1. In the first method (Figure 1a), the coenzyme is regenerated electrochemically using electrons supplied from an electrode. To improve efficiency, a regeneration enzyme and chemical mediators are added to more effectively interact with the electrode. The second method (Figure 1b) does not use an electrode and instead uses a second enzyme that catalyses a reverse reaction to regenerate coenzyme. Use of a second enzyme reaction requires close coupling of the reaction kinetics of the secondary enzyme to match that of the primary enzyme reaction.
For example, if the rate of catalysis of the primary reaction is 500 mmol min-1 and the secondary enzyme catalyses at a rate of 200 mmol min-1, then the slower reaction will drag the primary reaction and lower the efficiency. On the other hand, if the secondary reaction is too fast (e.g. 2000 mmol min-1), then there would be inefficient and uneconomical waste of material. There are enzymes available that have been adapted for coenzyme regeneration to improve the efficiency of the process.
Many attempts have been made to immobilise coenzymes and enzymes together to try to produce a universal efficient biocatalytic system with continuous coenzyme regeneration and, although some systems work well, further innovation is still required to produce a fully efficient and effective system that will realise the full potential of all the valuable enzymes that have coenzyme requirements.
 الاكثر قراءة في الانزيمات
الاكثر قراءة في الانزيمات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















