

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Enzymes
المؤلف:
John M Walker and Ralph Rapley
المصدر:
Molecular Biology and Biotechnology 5th Edition
الجزء والصفحة:
4-1-2021
2322
Enzymes
Enzymes are a group of proteins that are synthesised by living cells to function as catalysts for the many thousands of biochemical reactions that constitute the metabolism of a cell. More than 2500 different enzymes are known and there are still many organisms that have not been screened for novel enzymes or metabolic pathways. It is likely that there are many enzymes awaiting discovery. A new addition to the designated list of enzymes in 2007 was the enzyme chlorophyllase (EC 3.1.1.14), which is part of the chlorophyll degradation pathway that is responsible for de-greening processes that occur during fruit ripening and flowering. The enzyme hydrolyses chlorophyll to phytol+chlorophyllide.
Living cells require enzymes to drive metabolic pathways because at physiological temperature and pH, uncatalysed reactions would proceed at too slow a rate for the vital processes necessary to sustain life. A list of the reaction types catalysed by enzymes is provided in Table 1. In common with all catalysts, enzymes are subject to the normal laws concerning catalysis of reactions:
- A catalyst cannot speed up a reaction that would not occur in its absence, because it is not thermodynamically possible.
- A catalyst is not consumed during the reaction and so relatively few catalyst molecules are capable of catalysing a reaction many times.
- A catalyst cannot alter the equilibrium position of a given reaction.
Most reactions proceed eventually to a state of equilibrium, in which the rate of the forward reaction is equal to the rate of the reverse reaction. At equilibrium the substrate and product have specific equilibrium concentrations that are a special characteristic of the reaction.
For example, isomerisation of glucose (substrate)
Table .1 Reactions types catalysed by enzymes.a
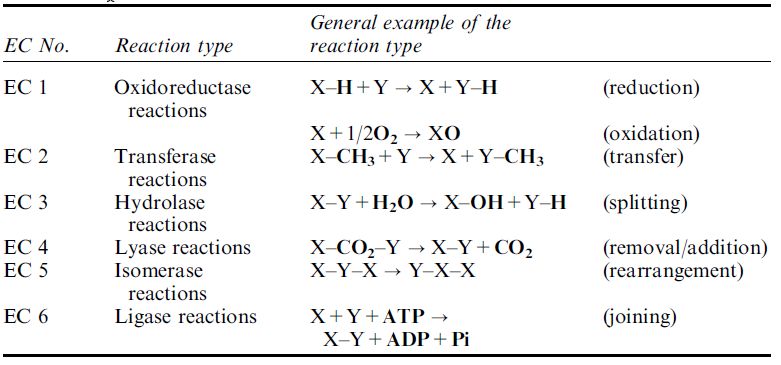
aEnzyme reaction types were grouped into six main families by an Enzyme Commission (EC) and underneath each main family there are sub-classes and sub-sub-classes that further describe details of the reaction catalysed. Each enzyme receives an EC number that provides details of the reaction catalysed. For example, the EC number for the enzyme lactate dehydrogenase is EC 1.1.1.27: The first number(1) indicates the enzyme is an oxidoreductase. The second number(1) indicates a sub-class that specifies CH–OH as a group involved in the reaction. The third number(1) indicates a sub-section that specifies NAD as the coenzyme used in the reaction. The fourth number(27) indicates the 27th enzyme in this sub-section that specifies L-lactate as the substrate for the reaction (note there are over 290 enzymes in this sub-section).
to produce fructose (product) is catalysed by the enzyme glucose isomerase:

From a starting solution of 100% glucose, the reaction proceeds to equilibrium and for this reaction the relative proportions at equilibrium are 45% fructose and 55% glucose. The enzyme cannot change the equilibrium position of the reaction, but it can substantially reduce the time that the reaction normally takes to reach equilibrium. In these respects, enzymes are no different from other catalysts. However, enzymes do possess two special attributes that are not found to any great extent in other non-biological catalysts: specificity and high catalytic power.
1.1 Specificity
Specificity is a distinctive feature of enzymes. Chemical catalysts have limited specificity, whereas enzymes have strong specificity for reactants (substrates), and for the susceptible bond involved in a reaction. The degree of specificity can vary from absolute to fairly broad. For example, the enzyme urease is absolutely specific for its substrate urea (NH2CONH2) and an almost identical structural analogue such as thiourea (NH2SONH2) is not hydrolysed . In contrast, the enzyme hexokinase is less specific and has group specificity for a small set of related sugar molecules:
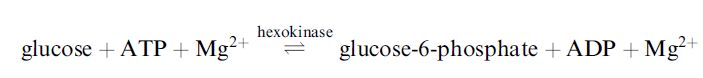
Glucose is the principal substrate. Hexokinase will also catalyse phosphorylation of several other sugars such as mannose and fructose, but not galactose, xylose, maltose or sucrose. In addition to substrate specificity, enzymes display remarkable product specificity, which ensures that the final product is not contaminated with by-products.
Thus, in the above phosphorylation of glucose, the product is exclusively glucose-6-phosphate and no other phosphoglucose (e.g. glucose-1-phosphate or glucose-2-phosphate) is produced during the reaction. The formation of by-products by side reactions is a significant problem associated with most of the less specific catalysts. In many enzymes, specificity also extends to selective discrimination between stereoisomers of a substrate molecule. This stereospecificity is shown by the enzyme Damino acid oxidase, which is specific for D-amino acids only and will not catalyse the oxidation of the L-amino acid stereoisomers.
Specificity is an inherent feature of enzymes because catalysis takes place in a particular region of the enzyme, which is designed to accommodate the substrates involved in a reaction (Figure 1). This
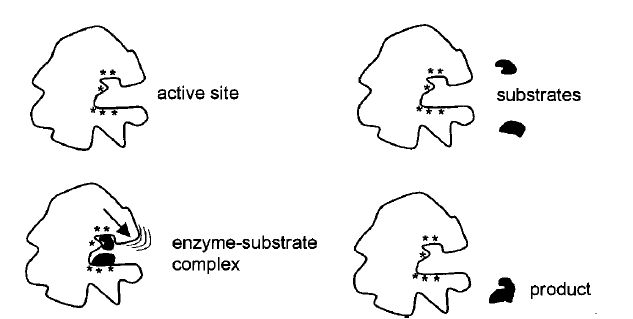
Figure .1 Schematic representation of an enzyme active site with amino acid residues (*) located at the active site, binding of substrates and change in enzyme shape after substrates are bound.
region or active site is normally a small pocket, cleft or crevice on the surface of the enzyme. It is designed to bring a few of the enzyme amino acid residues into contact with the substrate molecule. The active site has strong affinity for the substrate because site amino acid residues are primed for interaction with groups or atoms on the substrate molecule.
Consequently, a substrate molecule must have the correct shape with groups/atoms in the correct position to fit into the active site and participate in the interactions (Figure 1). Enzymes with absolute specificity have very precise shape/interaction requirements, which are only found in one particular substrate molecule. Enzymes with broad specificity have active site requirements that are more flexible and therefore accept a wider range of substrate molecules. The active site amino acid residues participate directly in a catalytic reaction and are largely responsible for the high catalytic power associated with enzyme reactions.
1.2 Catalytic Power
During any reaction, the reactants briefly enter a state in which susceptible substrate bonds are not completely broken and new bonds in the product are not completely formed. This temporary condition is called a transition state and is energy dependent because energy is needed to make and break chemical bonds (e.g. 350 kJ mol-1 for each C–C covalent bond) in substrate and product molecules. Formation of a transition state represents an energy barrier to successful reaction and is the reason why most reactions proceed extremely slowly in the absence of external help.
Substrates can be helped towards a transition state by addition of heat energy, high pressure or extreme pH to weaken bonds or by addition of catalysts. Enzymes are very effective at reducing the energy barrier to allow the formation of a transition state and increased the rate of a reaction. The efficiency of enzyme catalysis varies, but most enzymes can enhance the rate of an uncatalysed reaction by a factor in the range 105–1014. One of the most efficient enzymes is carbonic anhydrase, which catalyses the hydration of up to 600 000 molecules of CO2 per second under optimal conditions:
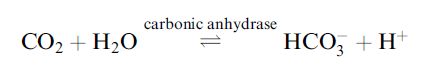
Carbonic anhydrase has a vital role in maintaining blood pH at 7.4 and in removal of CO2 from blood by gas exchange in the lungs. In medical conditions/emergencies when breathing has stopped or is difficult and intervention to remove CO2 is required, it is normally achieved with assistance of mechanical ventilators. When lung damage/dysfunction is apparent, then ventilator assistance can harm weakened lungs and in such circumstances there is a need for a breathing assistance device that is independent of the lungs. Core to the development of such
a device is immobilisation of carbonic anhydrase and recent studies have demonstrated the use of hollow-fibre membranes (HFMs) with immobilised carbonic anhydrase to enhance CO2 exchange in a respiratory assist device as depicted in Figure .2.
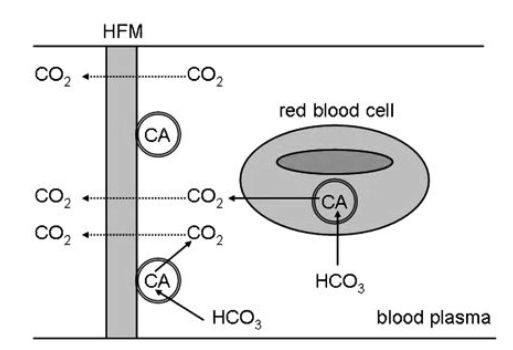
Figure .2 Schematic of a CO2 removal assist device based on a hollow-fibre membrane (HFM) containing bound carbonic anhydrase (CA) for conversion of bicarbonate (HCO3) to CO2 for removal through the membrane.
 الاكثر قراءة في الانزيمات
الاكثر قراءة في الانزيمات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















