

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Chirality in polymer
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
26-5-2020
1123
Chirality in polymer
The tetrahedral nature of carbon bonding has an important consequence that is not revealed by simple two-dimensional structural formulas: atoms attached to the carbon can be on one side or on the other, and these will not be geometrically equivalent if all four of the groups attached to a single carbon atom are different. Such carbons (and the groups attached to them) are said to be chiral, and can exist in two different three-dimensional forms known as enantiomers.
For an individual carbon atom in a polymer chain, two of its attached groups will ordinarily be the chain segments on either side of the carbon. If the two remaining groups are different (say one hydrogen and the other methyl), then the above conditions are satisfied and this part of the chain can give rise to two enantiomeric forms.
A chain that can be represented as (in which the orange and green circles represent different groups) will have multiple chiral centers, giving rise to a huge number of possible enantiomers. In practice, it is usually sufficient to classify chiral polymers into the following three classes of stereoregularity, usually referred to as tacticity.
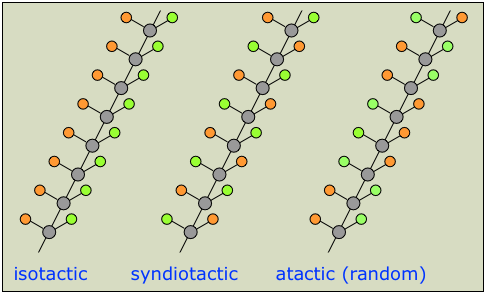
The tacticity of a polymer chain can have a major influence on its properties. Atactic polymers, for example, being more disordered, cannot crystallize.
One of the major breakthroughs in polymer chemistry occurred in the early 1950s when the German chemist Karl Ziegler discovered a group of catalysts that could efficiently polymerize ethylene. At about the same time, Giulio Natta (Italian) made the first isotactic (and crystalline) polyethylene. The Ziegler-Natta catalysts revolutionized polymer chemistry by making it possible to control the stereoregularity of these giant molecules. The two shared the 1963 Nobel Prize in Chemistry
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















