

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Amorphous and crystalline polymers
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
26-5-2020
1338
Amorphous and crystalline polymers
The spaghetti-like entanglements of polymer molecules tend to produce amorphous solids, but it often happens that some parts can become sufficiently aligned to produce a region exhibiting crystal-like order, so it is not uncommon for some polymeric solids to consist of a random mixture of amorphous and crystalline regions. As might be expected, shorter and less-branched polymer chains can more easily organize themselves into ordered layers than have can long chains. Hydrogen-bonding between adjacent chains also helps, and is very important in fiber-forming polymers both synthetic (Nylon 6.6) and natural (cotton cellulose).
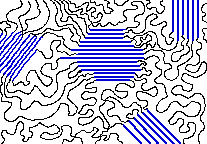
Figure 1: The crystalline parts of this polymer are shown in blue.
Pure crystalline solids have definite melting points, but polymers, if they melt at all, exhibit a more complex behavior. At low temperatures, the tangled polymer chains tend to behave as rigid glasses. For example, the natural polymer that we call rubber becomes hard and brittle when cooled to liquid nitrogen temperature. Many synthetic polymers remain in this state to well above room temperature.
In some polymers (known as thermoplastics) there is a fairly definite softening point that is observed when the thermal kinetic energy becomes high enough to allow internal rotation to occur within the bonds and to allow the individual molecules to slide independently of their neighbors, thus rendering them more flexible and deformable. This defines the glass transition temperature tg .
Depending on the degree of crystallinity, there will be a higher temperature, the melting point tm , at which the crystalline regions come apart and the material becomes a viscous liquid. Such liquids can easily be injected into molds to manufacture objects of various shapes, or extruded into sheets or fibers. Other polymers (generally those that are highly cross-linked) do not melt at all; these are known as thermosets. If they are to be made into molded objects, the polymerization reaction must take place within the molds — a far more complicated process. About 20% of the commercially-produced polymers are thermosets; the remainder are thermoplastics.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















