

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Monosubstituted Cyclohexanes
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
4-7-2019
1945
Monosubstituted Cyclohexanes
The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction.
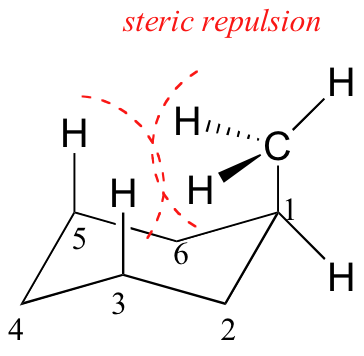
The relative steric hindrance experienced by different substituent groups oriented in an axial versus equatorial location on cyclohexane may be determined by the conformational equilibrium of the compound. The corresponding equilibrium constant is related to the energy difference between the conformers, and collecting such data allows us to evaluate the relative tendency of substituents to exist in an equatorial or axial location. Table 1.1
show some of these free energy values (sometimes referred to as A values).
| Substituent Group | ΔG º (axial–equatorial) | Substituent Group | ΔG º (axial–equatorial) |
|---|---|---|---|
| CH3 | 1.74 kcal mol-1 | F | 0.3 |
| C2H5 | 1.8 | Cl | 0.6 |
| (CH3)2CH | 2.2 | Br | 0.6 |
| (CH3)3C | 4.7 | I | 0.55 |
| CF3 | 2.4 | OH | 0.6 - 1.0 |
| C6H5 | 2.8 | OCH3 | 0.65 |
| CH2=CH | 1.6 | OCOCH3 | 0.75 |
| HC≡C | 0.45 | OSI(CH3)3 | 0.7 |
| CN | 0.2 | SH | 1.2 |
| CHO | 0.7 | NH2 | 1.3 - 1.7 |
| COCH3 | 1.2 | N(CH3)2 | 2.0 |
| CO2H | 1.4 | N3 | 0.5 |
| CO2 Na | 2.0 | NO2 | 1.1 |
| CO2CH3 | 1.3 | Si(CH3)3 | 2.5 |
| COCl | 1.3 | ||
| These "Conformational Energies" or "A values" are -ΔG° 's for axial/equatorial equilibria of substituted cyclohexanes. The data in this table are averaged from several different sources. For a more precise and extensive compilation see: E.L.Eliel and S.H.Wilen, Stereochemistry of Organic Compounds, 1994. | |||
Looking at the energy values in Table 1.1 , it is clear that the apparent "size" of a substituent (in terms of its preference for equatorial over axial orientation) is influenced by its width and bond length to cyclohexane, as evidenced by the fact that an axial vinyl group is less hindered than ethyl, and iodine slightly less than chlorine.
We noted earlier that cycloalkanes having two or more substituents on different ring carbon atoms exist as a pair (sometimes more) of configurational stereoisomers. Now we must examine the way in which favorable ring conformations influence the properties of the configurational isomers. Remember, configurational stereoisomers are stable and do not easily interconvert, whereas, conformational isomers normally interconvert rapidly. In examining possible structures for substituted cyclohexanes, it is useful to follow two principles:
- Chair conformations are generally more stable than other possibilities.
- Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















