

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Nitrogen
المؤلف:
........
المصدر:
LibreTexts Project
الجزء والصفحة:
............
19-10-2018
1571
Nitrogen
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with its self, and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before 100 years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food, and as a fertilizer.
Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Molecular nitrogen (N2
) is not reactive at standard temperature and pressure and is a colorless and odorless gas.
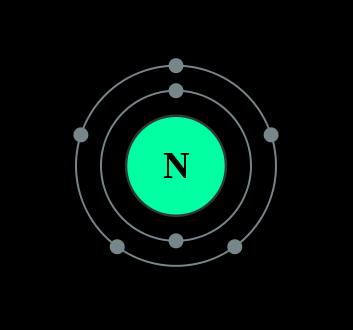
Figure 1.1
: A Bohr diagram of the nitrogen atom.
Nitrogen is a non-metal element that occurs most abundantly in the atmosphere, nitrogen gas (N2) comprises 78.1% of the volume of the Earth’s air. It only appears in 0.002% of the earth's crust by mass. Compounds of nitrogen are found in foods, explosives, poisons, and fertilizers. Nitrogen makes up DNA in the form of nitrogenous bases as well as in neurotransmitters. It is one of the largest industrial gases, and is produced commercially as a gas and a liquid.
| Name and Symbol | Nitrogen, N |
| Category | non-metal |
| Atomic Weight | 14.0067 |
| Group | 15 |
| Electron Configuration | 1s2 2s2 2p3 |
| Valence Electrons | 2, 5 |
| Phase | Gas |
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















