
Nucleophilic Substitution of the Hydroxyl Group
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 19-9-2018
19-9-2018
 1388
1388
Nucleophilic Substitution of the Hydroxyl Group
Using the chemical behavior of alkyl halides as a reference, we are encouraged to look for analogous substitution and elimination reactions of alcohols. The chief difference, of course, is a change in the leaving anion from halide to hydroxide. Since oxygen is slightly more electronegative than chlorine (3.5 vs. 2.8 on the Pauling scale), we expect the C-O bond to be more polar than a C-Cl bond. Furthermore, an independent measure of the electrophilic character of carbon atoms from their nmr chemical shifts (both 13C & alpha protons), indicates that oxygen and chlorine substituents exert a similar electron-withdrawing influence when bonded to sp3 hybridized carbon atoms. Despite this promising background evidence, alcohols do not undergo the same SN2 reactions commonly observed with alkyl halides. For example, the rapid SN2 reaction of 1-bromobutane with sodium cyanide, shown below, has no parallel when 1-butanol is treated with sodium cyanide. In fact ethyl alcohol is often used as a solvent for alkyl halide substitution reactions such as this.
CH3CH2CH2CH2–Br + Na(+) CN(–)  CH3CH2CH2CH2–CN + Na(+) Br(–) CH3CH2CH2CH2–CN + Na(+) Br(–) |
CH3CH2CH2CH2–OH + Na(+) CN(–)  No Reaction No Reaction |
The key factor here is the stability of the leaving anion (bromide vs. hydroxide). We know that HBr is a much stronger acid than water (by more than 18 powers of ten), and this difference will be reflected in reactions that generate their conjugate bases. The weaker base, bromide anion, is more stable and its release in a substitution or elimination reaction will be much more favorable than that of hydroxide ion, a stronger and less stable base.
Clearly, an obvious step toward improving the reactivity of alcohols in SN2 reactions would be to modify the –OH functional group in a way that improves its stability as a leaving anion. One such modification is to conduct the substitution reaction in strong acid so that –OH is converted to –OH2(+). Since the hydronium ion (H3O(+)) is a much stronger acid than water, its conjugate base (H2O) is a better leaving group than hydroxide ion. The only problem with this strategy is that many nucleophiles, including cyanide, are deactivated by protonation in strong acid, effectively removing the nucleophilic co-reactant needed for the substitution. The strong acids HCl, HBr and HI are not subject to this difficulty because their conjugate bases are good nucleophiles and are even weaker bases than alcohols. The following equations illustrate some substitution reactions of alcohols that may be effected by these acids. As was true for alkyl halides, nucleophilic substitution of 1º-alcohols proceeds by an SN2 mechanism, whereas 3º-alcohols react by an SN1 mechanism. Reactions of 2º-alcohols may occur by both mechanisms and often produce some rearranged products. The numbers in parentheses next to the mineral acid formulas represent the weight percentage of a concentrated aqueous solution, the form in which these acids are normally used.
CH3CH2CH2CH2–OH + HBr (48%)  CH3CH2CH2CH2–OH2(+) Br(–) CH3CH2CH2CH2–OH2(+) Br(–)  CH3CH2CH2CH2–Br + H2O SN2 CH3CH2CH2CH2–Br + H2O SN2 |
(CH3)3C–OH + HCl (37%)  (CH3)3C–OH2(+) Cl(–) (CH3)3C–OH2(+) Cl(–)  (CH3)3C(+) Cl(–) + H2O (CH3)3C(+) Cl(–) + H2O  (CH3)3C–Cl + H2O SN1 (CH3)3C–Cl + H2O SN1 |
Although these reactions are sometimes referred to as "acid-catalyzed" this is not strictly correct. In the overall transformation a strong HX acid is converted to water, a very weak acid, so at least a stoichiometric quantity of HX is required for a complete conversion of alcohol to alkyl halide. The necessity of using equivalent quantities of very strong acids in this reaction limits its usefulness to simple alcohols of the kind shown above. Alcohols having acid sensitive groups would, of course, not tolerate such treatment. Nevertheless, the idea of modifying the -OH functional group to improve its stability as a leaving anion can be pursued in other directions. The following diagram shows some modifications that have proven effective. In each case the hydroxyl group is converted to an ester of a strong acid. The first two examples show the sulfonate esters described earlier. The third and fourth examples show the formation of a phosphite ester (X represents remaining bromines or additional alcohol substituents) and a chlorosulfite ester respectively. All of these leaving groups (colored blue) have conjugate acids that are much stronger than water (by 13 to 16 powers of ten) so the leaving anion is correspondingly more stable than hydroxide ion. The mesylate and tosylate compounds are particularly useful in that they may be used in substitution reactions with a wide variety of nucleophiles. The intermediates produced in reactions of alcohols with phosphorus tribromide and thionyl chloride (last two examples) are seldom isolated, and these reactions continue on to alkyl bromide and chloride products.
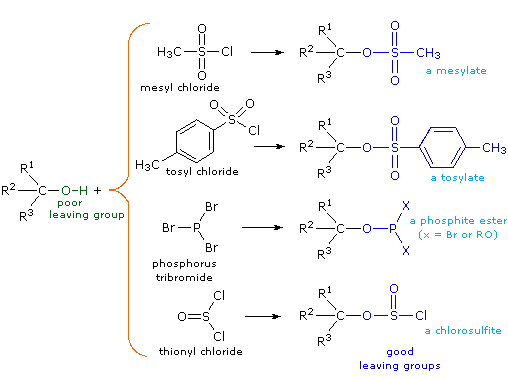
The importance of sulfonate ester intermediates in general nucleophilic substitution reactions of alcohols may be illustrated by the following conversion of 1-butanol to pentanenitrile (butyl cyanide), a reaction that does not occur with the alcohol alone (see above). The phosphorus and thionyl halides, on the other hand, only act to convert alcohols to the corresponding alkyl halides.
| CH3CH2CH2CH2–OH + CH3SO2Cl |
pyridine

|
CH3CH2CH2CH2–OSO2CH3 |
Na(+) CN(–)

|
CH3CH2CH2CH2–CN + CH3SO2O(–) Na(+) |
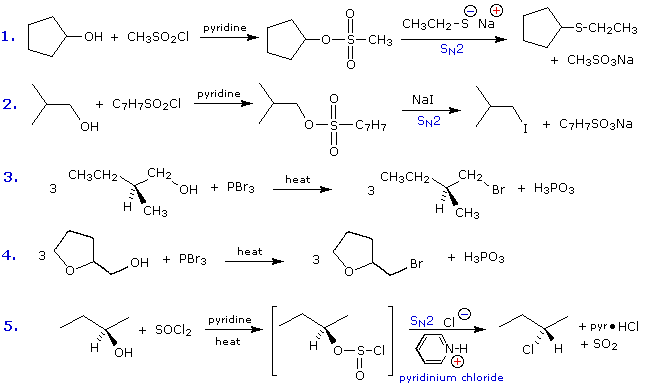
Some examples of alcohol substitution reactions using this approach to activating the hydroxyl group are shown in the following diagram. The first two cases serve to reinforce the fact that sulfonate ester derivatives of alcohols may replace alkyl halides in a variety of SN2 reactions. The next two cases demonstrate the use of phosphorus tribromide in converting alcohols to bromides. This reagent may be used without added base (e.g. pyridine), because the phosphorous acid product is a weaker acid than HBr. Phosphorous tribromide is best used with 1º-alcohols, since 2º-alcohols often give rearrangement by-products resulting from competing SN1 reactions. Note that the ether oxygen in reaction 4 is not affected by this reagent; whereas, the alternative synthesis using concentrated HBr cleaves ethers. Phosphorus trichloride (PCl3) converts alcohols to alkyl chlorides in a similar manner, but thionyl chloride is usually preferred for this transformation since the inorganic products are gases (SO2 & HCl). Phosphorus triiodide is not stable, but may be generated in situ from a mixture of red phosphorus and iodine, and acts to convert alcohols to alkyl iodides. The last example shows the reaction of thionyl chloride with a chiral 2º-alcohol. The presence of an organic base such as pyridine is important, because it provides a substantial concentration of chloride ion needed for the final SN2 reaction of the chlorosufite intermediate. In the absence of base chlorosufites decompose on heating to give the expected alkyl chloride with retention of configuration
Tertiary alcohols are not commonly used for substitution reactions of the kind discussed here, because SN1 and E1 reaction paths are dominant and are difficult to control. This aspect of alcohol chemistry will be touched upon in the next section.
The importance of sulfonate esters as intermediates in many substitution reactions cannot be overstated. A rigorous proof of the configurational inversion that occurs at the substitution site in SN2 reactions makes use of such reactions. An example of such a proof will display above when the An Inversion Proof button beneath the diagram is pressed. Abbreviations for the more commonly used sulfonyl derivatives are given in the following table.
| Sulfonyl Group |
CH3SO2– |
CH3C6H4SO2– |
BrC6H4SO2– |
CF3SO2– |
| Name & Abbrev. |
Mesyl or Ms |
Tosyl or Ts |
Brosyl or Bs |
Trifyl or Tf |
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


