

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Cannizzaro Reaction
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
5-8-2018
3619
The Cannizzaro Reaction
When a non-enolizable aldehyde is heated in strong aqueous base, a redox transformation known as the Cannizzaro reaction takes place. Two examples are shown in the following diagram. In the first, formaldehyde disproportionates into methanol and formic acid (sodium salt). In the second, a benzaldehyde derivative is similarly converted into an equimolar mixture of the corresponding benzyl alcohol and benzoic acid derivatives. A hydride transfer mechanism analogous to that of the MVP reaction is drawn in the shaded box. If the Cannizzaro reaction is run in D2O with NaOD as a base, no C-D incorporation is observed. Thus the new carbon-bonded hydrogen in the alcohol cannot have come from the solvent.
It is important to remember that the the Cannizzaro reaction is restricted to non-enolizable aldehydes. The strong base used for this reaction would initiate aldol and other reactions that take place via enolate anions. A useful crossed Cannizzaro reaction employs an excess of formaldehyde to reduce aryl aldehyde substrates to 1 º-alcohols. The success of this procedure may be attributed to the high concentration of the hydrate, H2C(OH)2, in aqueous solutions of formaldehyde, making it the only significant hydride donor in the system.

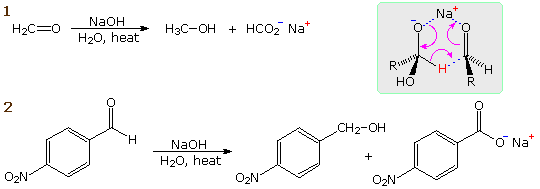
An intramolecular Cannizzaro reaction, sometimes termed a Cannizzaro rearrangement will be displayed above by clicking on the diagram. A variant of the Cannizzaro reaction, known as the Tischenko reaction is also shown. In this reaction the alcohol and acid products combine to form an ester.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















