

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Alkyl Moiety
المؤلف:
Charge DistributionWilliam Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
28-7-2018
1538
The Alkyl Moiety
Some of the most important information concerning nucleophilic substitution and elimination reactions of alkyl halides has come from studies in which the structure of the alkyl group has been varied. If we examine a series of alkyl bromide substitution reactions with the strong nucleophile thiocyanide (SCN) in ethanol solvent, we find large decreases in the rates of reaction as alkyl substitution of the alpha-carbon increases. Methyl bromide reacts 20 to 30 times faster than simple 1º-alkyl bromides, which in turn react about 20 times faster than simple 2º-alkyl bromides, and 3º-alkyl bromides are essentially unreactive or undergo elimination reactions. Furthermore, β-alkyl substitution also decreases the rate of substitution, as witnessed by the failure of neopentyl bromide, (CH3)3CCH2-Br (a 1º-bromide), to react.
Alkyl halides in which the alpha-carbon is a chiral center provide additional information about these nucleophilic substitution reactions. Returning to the examples presented at the beginning of this section, we find that reactions 2, 5 & 6 demonstrate an inversion of configuration when the cyanide nucleophile replaces the bromine.
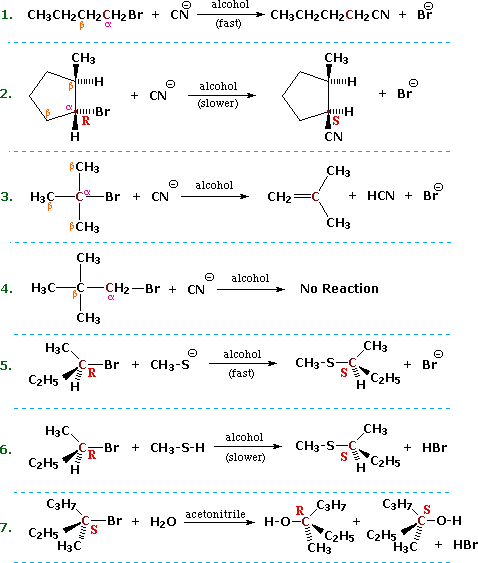
Other investigations have shown this to be generally true for reactions carried out in non-polar organic solvents, the reaction of (S)-2-iodobutane with sodium azide in ethanol being just one example ( in the following equation the alpha-carbon is maroon and the azide nucleophile is blue). Inversion of configuration during nucleophilic substitution has also been confirmed for chiral 1º-halides of the type RCDH-X, where the chirality is due to isotopic substitution.
(S)-CH3CHICH2CH3 + NaN3 ——> (R)-CH3CHN3CH2CH3 + NaI
We can now piece together a plausible picture of how nucleophilic substitution reactions of 1º and 2º-alkyl halides take place. The nucleophile must approach the electrophilic alpha-carbon atom from the side opposite the halogen. As a covalent bond begins to form between the nucleophile and the carbon, the carbon halogen bond weakens and stretches, the halogen atom eventually leaving as an anion. The diagram on the rightbelow shows this process for an anionic nucleophile.
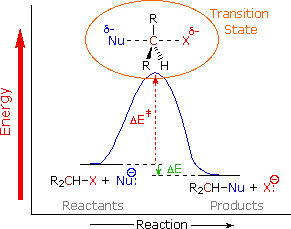
We call this description the SN2 mechanism, where S stands for Substitution, N stands for Nucleophilic and 2 stands for bimolecular (defined below). In the SN2 transition state the alpha-carbon is hybridized sp2 with the partial bonds to the nucleophile and the halogen having largely p-character. Both the nucleophile and the halogen bear a partial negative charge, the full charge being transferred to the halogen in the products. The consequence of rear-side bonding by the nucleophile is an inversion of configuration about the alpha-carbon. Neutral nucleophiles react by a similar mechanism, but the charge distribution in the transition state is very different.
This mechanistic model explains many aspects of the reaction. First, it accounts for the fact that different nucleophilic reagents react at very different rates, even with the same alkyl halide. Since the transition state has a partial bond from the alpha-carbon to the nucleophile, variations in these bond strengths will clearly affect the activation energy, ΔE‡, of the reaction and therefore its rate. Second, the rear-side approach of the nucleophile to the alpha-carbon will be subject to hindrance by neighboring alkyl substituents, both on the alpha and the beta-carbons. The following models clearly show this "steric hindrance" effect.
The two models displayed below start as methyl bromide, on the left, and ethyl bromide, on the right. These may be replaced by isopropyl, tert-butyl, neopentyl, and benzyl bromide models by pressing the appropriate buttons. (note that when first activated, this display may require clicking twice on the selected button.) In each picture the nucleophile is designated by a large violet sphere, located 3.75 Angstroms from the alpha-carbon atom (colored a dark gray), and located exactly opposite to the bromine (colored red-brown). This represents a point on the trajectory the nucleophile must follow if it is to bond to the back-side of the carbon atom, displacing bromide anion from the front face. With the exception of methyl and benzyl, the other alkyl groups present a steric hindrance to the back-side approach of the nucleophile, which increases with substitution alpha and beta to the bromine. The hydrogen (and carbon) atoms that hinder the nucleophile's approach are colored a light red. The magnitude of this steric hindrance may be seen by moving the models about in the usual way, and is clearly greatest for tert-butyl and neopentyl, the two compounds that fail to give substitution reactions.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















