

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hyperconjugation
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
20-7-2018
6179
Hyperconjugation
Another way in which alkyl substituents stabilize carbocations is illustrated in the following diagram. This conjugative charge delocalization, called hyperconjugation, involves partial pi-bond formation to alpha-carbon atoms, provided suitably oriented C-H or C-C bonds are present. The small increase in stability of the 1-propyl cation compared with an ethyl cation, as noted above, suggests that C-C hyperconjugation provides slightly greater stabilization than does the C-H hyperconjugation shown here. Hyperconjugation by alkyl substituents also acts to stabilize unsaturated functional groups, as noted earlier for carbon-carbon double bonds.
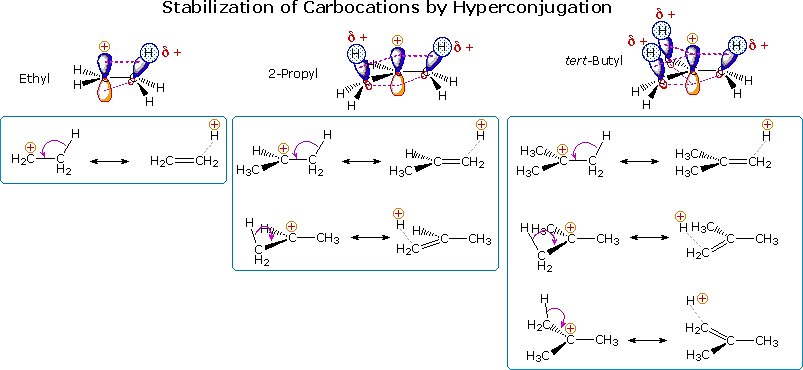
Since hyperconjugation and the inductive effect act in the same manner, their relative importance in carbocation stabilization is a matter of interest. Some insight to this question is found in a group of novel compounds, bridgehead substituted bicyclic halides. Two examples of these compounds are shown below. The nomenclature of bridged bicyclic compounds identifies the length of the chains that connect the bridgehead atoms (colored pink here). Three connecting chains are present in a bicyclic compound, and the number of atoms in each chain (excluding hydrogen) is given as a number in brackets. If the chains are of different lengths the longest is listed first. One of the bridgehead atoms is numbered one, and the longest chain continues the numbering sequence until the second bridgehead atom is included. Numbering then continues along the next longest chain. The base name of the bicyclic compound reflects the total number of carbons, and is therefore the sum of the bridging chains plus two (the bridgehead atoms).
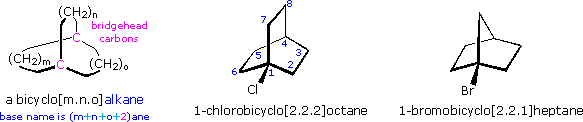
The bridgehead substituted halides shown above will form 3º-carbocations when ionized. Inductive stabilization of these cations should be similar to that of the tert-butyl cation, so if this were the predominant stabilizing factor from alkyl substitution, the reactivity of these halides should be similar to their tert-butyl counterparts. In practice, however, the bridgehead halides were found to be much less reactive. Indeed, 1-chlorobicyclo[2.2.1]heptane was recovered unchanged from prolonged treatment with hot ethanolic silver nitrate. The instability of such bridgehead carbocations has been attributed to the pyramidal shape forced upon the trigonal carbon (sp2 hybridized). However, covalent bonds are generally able to accommodate modest bending distortions without significant destabilization, and the inductive shift of electron pairs toward the positively charged carbon atom is unlikely to be impeded by a pyramidal configuration of the carbocation.
An alternative explanation is that hyperconjugation with the alpha-methylene groups is prohibited by the rigid configuration of these bridgehead cations. By clicking on the diagram above, an illustration of one possible C-H hyperconjugative interaction will be displayed. The carbon-carbon double bond implicit to this occurrence is badly twisted, and could not exist as such in any stable alkene. Structural prohibitions of this kind are encompassed in an empirical guideline called Bredt's rule.
On the other hand, C-C hyperconjugation may act to partially stabilize bridgehead carbocations. By clicking on the diagram a second time, two examples of such hyperconjugation will appear. While still relatively inert, the bicyclo[2.2.2]octane compound is roughly a million times more reactive than its bicyclo[2.2.1]heptane analog, shown above. This may be attributed to improved hyperconjugation, since the appropriate C-C bonds (colored red) are better aligned with a developing bridgehead carbocation. Furthermore, confirmation of expected changes in bond lengths resulting from such hyperconjugation has been obtained by X-ray diffraction analysis of a crystalline SbF6 salt of the adamantane cation. In this study, the red-colored bonds were lengthened and the green-colored bonds were shortened.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















