
Avogadro’s Law: The amount of gas
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p 168
الجزء والصفحة:
p 168
 31-1-2017
31-1-2017
 1699
1699
Avogadro’s Law: The amount of gas
Amedeo Avogadro determined, from his study of gases, that equal volumes of gases at the same temperature and pressure contain equal numbers of gas particles. So Avogadro’s law says that the volume of a gas is directly proportional to the number of moles of gas (number of gas particles) at a constant temperature and pressure. Mathematically, Avogadro’s law looks like this: V = kn (at constant temperature and pressure) In this equation, k is a constant and n is the number of moles of gas. If you have a number of moles of gas (n1) at one volume (V1), and the moles change due to a reaction (n2), the volume also changes (V2), giving you the equation
V1/n1 = V2/n2
A very useful consequence of Avogadro’s Law is that you can calculate the volume of a mole of gas at any temperature and pressure. An extremely useful form to know when calculating the volume of a mole of gas is that 1 mole of any gas at STP occupies 22.4 liters. STP in this case is not an oil or gas additive; it stands for standard temperature and pressure.
✓ Standard pressure: 1.00 atm (760 torr or mm Hg)
✓ Standard temperature: 273 K
This relationship between moles of gas and liters gives you a way to convert the gas from a mass to a volume. For example, suppose that you have 50.0 grams of oxygen gas (O2) and you want to know its volume at STP. You can set up the problem like this
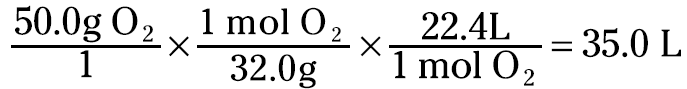
You now know that the 50.0 grams of oxygen gas occupies a volume of 35.0 liters at STP.
If the gas isn’t at STP, you can use the combined gas law (from the preceding section) to find the volume at the new pressure and temperature — or you can use the ideal gas equation, which I show you next.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


