
Enthalpies of physical change
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص50-51
الجزء والصفحة:
ص50-51
 2025-11-02
2025-11-02
 41
41
Enthalpies of physical change
The standard enthalpy change that accompanies a change of physical state is called the standard enthalpy of transition and is denoted ∆trsH ْ(Table 2.3). The standard enthalpy of vaporization, ∆vapH ْ, is one example. Another is the standard enthalpy of fusion, ∆fusH ْ, the standard enthalpy change accompanying the conversion of a solid to a liquid, as in
H2O(s) →H2O(l) ∆fusH ْ (273 K) =+6.01 kJ mol−1
As in this case, it is sometimes convenient to know the standard enthalpy change at the transition temperature as well as at the conventional temperature. Because enthalpy is a state function, a change in enthalpy is independent of the path between the two states. This feature is of great importance in thermochemistry, for it implies that the same value of ∆H ْ will be obtained however the change is brought about between the same initial and final states. For example, we can picture the con version of a solid to a vapour either as occurring by sublimation (the direct conversion from solid to vapour), H2O(s) →H2O(g) ∆subH ْ or as occurring in two steps, first fusion (melting) and then vaporization of the resulting liquid:
H2O(s) →H2O(l) ∆fusH ْ
H2O(l) →H2O(g) ∆vapH ْ
Overall: H2O(s) → H2O(g) ∆fusH ْ +∆vapH ْ
Because the overall result of the indirect path is the same as that of the direct path, the overall enthalpy change is the same in each case (1), and we can conclude that (for processes occurring at the same temperature)
∆subH ْ =∆fusH ْ +∆vapH ْ
An immediate conclusion is that, because all enthalpies of fusion are positive, the enthalpy of sublimation of a substance is greater than its enthalpy of vaporization (at a given temperature). Another consequence of H being a state function is that the standard enthalpy changes of a forward process and its reverse differ in sign (2):
∆H ْ (A→B) =−∆H ْ (B→A)
For instance, because the enthalpy of vaporization of water is +44 kJ mol−1 at 298 K, its enthalpy of condensation at that temperature is −44 kJ mol−1.
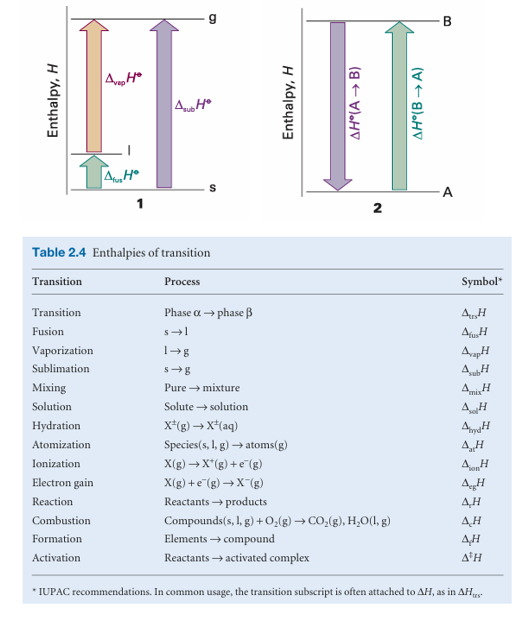
The different types of enthalpies encountered in thermochemistry are summarized in Table 2.4. We shall meet them again in various locations throughout the text.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


