
The measurement of an enthalpy change
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص41-44
الجزء والصفحة:
ص41-44
 2025-11-02
2025-11-02
 45
45
The measurement of an enthalpy change
An enthalpy change can be measured calorimetrically by monitoring the temperature change that accompanies a physical or chemical change occurring at constant pressure. A calorimeter for studying processes at constant pressure is called an isobaric calorimeter. A simple example is a thermally insulated vessel open to the atmosphere: the heat released in the reaction is monitored by measuring the change in temperature of the contents. For a combustion reaction an adiabatic flame calorimeter may be used to measure ∆T when a given amount of substance burns in a supply of oxygen (Fig. 2.13). Another route to ∆His to measure the internal energy change by using a bomb calorimeter, and then to convert ∆U to ∆H. Because solids and liquids have small molar volumes, for them pVmis so small that the molar enthalpy and molar internal energy are almost identical (Hm=Um+pVm≈Um). Consequently, if a process involves only solids or liquids, the values of ∆H and ∆U are almost identical. Physically, such processes are accompanied by a very small change in volume, the system does negligible work on the surroundings when the process occurs, so the energy supplied as heat stays entirely within the system. The most sophisticated way to measure enthalpy changes, however, is to use a differential scanning calorimeter (DSC). Changes in enthalpy and internal energy may also be measured by noncalori metric methods (see Chapter 7).

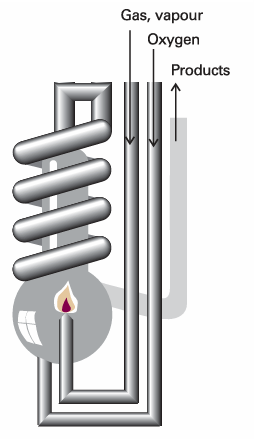
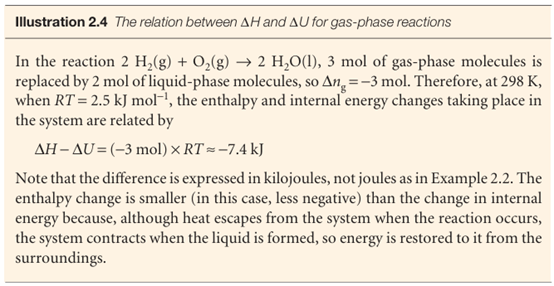
Fig. 2.13A constant-pressure flame calorimeter consists of this component immersed in a stirred water bath. Combustion occurs as a known amount of reactant is passed through to fuel the flame, and the rise of temperature is monitored. The enthalpy of a perfect gas is related to its internal energy by using pV=nRTin the definition of H: , H =U+pV=U+nRT , This relation implies that the change of enthalpy in a reaction that produces or consumes gas is
∆H=∆U+∆ng RT , Where ∆ng is the change in the amount of gas molecules in the reaction.


 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


