

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
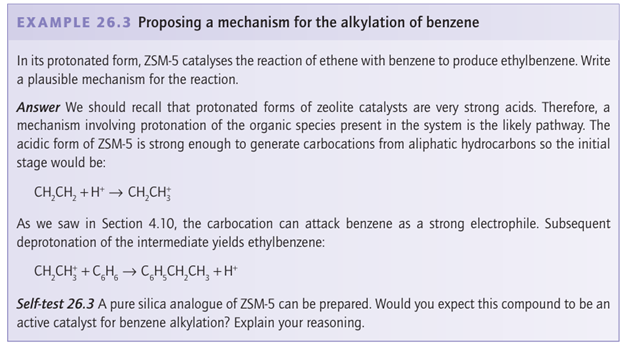
Catalytic cracking and the interconversion of aromatics by zeolites
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص710-713
2025-10-20
48
Catalytic cracking and the interconversion of aromatics by zeolites
Key points: Zeolite catalysts have strongly acidic sites that promote reactions such as isomerization via carbonium ions; shape selectivity may arise at various stages of the reaction due to the relative dimensions of the zeolite channels and reactant, intermediate, and product molecules. The zeolite-based (Sections 14.15 and 24.12) heterogeneous catalysts play an important role in the interconversion of hydrocarbons and the alkylation of aromatics as well as in oxidation and reduction. Two important zeolites used for such reactions are faujasite (Fig 26.22), also known as zeolite X or zeolite Y (the X or Y terminology is defined by the Si:Al ratio of the material, X has a higher Al content) and zeolite ZSM-5, an aluminosilicate
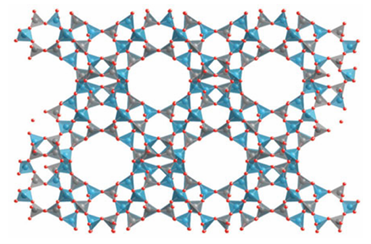
Figure 26.22 The zeolite faujasite (also known as zeolite X or Y) framework structure showing the large pore in which catalytic cracking occurs. Tetrahedra are SiO4 or AlO4.
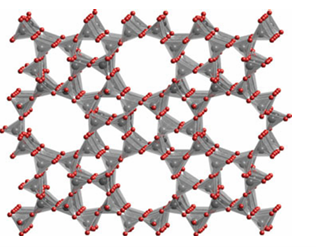
Figure 26.23 The zeolite ZSM-5 structure highlighting the channels along which small molecules may diffuse. Tetrahedra are SiO4 or AlO4.
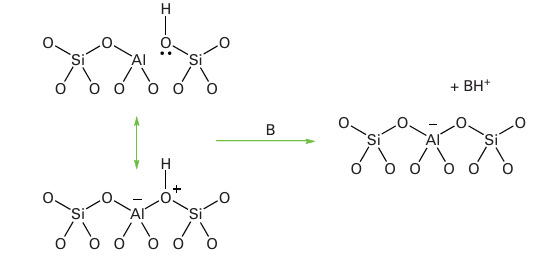
Figure 26.24 The Brønsted acid site in H-ZSM5 and its interaction with a base, typically an organic molecule. (Based on W.O. Haag, R.M. Lago, and P.B. Weisz, Nature, 1984, 309, 589.)
zeolite with a high Si content.5 The channels of ZSM-5 consist of a three-dimensional maze of intersecting tunnels (Fig. 26.23). As with other aluminosilicate catalysts, the Al sites are strongly acidic. The charge imbalance of Al3 in place of tetrahedrally coordinated Si(IV) requires the presence of an added positive ion. When this ion is H (Fig. 26.24) the Brønsted acidity of the aluminosilicate can be higher than that of concentrated H2 SO4 and is termed a superacid (Section 4.15); the turnover frequency for hydrocarbon reactions at these sites can be very high. Natural petroleum consists of only about 20 per cent of alkanes suitable for use in petrol (gasoline) and diesel with chain lengths ranging from C5 H12, pentane, to C12 H26 . Conversion of the higher molar mass hydrocarbons to the valuable lighter ones involves breaking the C-C bonds but also structural rearrangement of the hydrocarbons through dehydrogenation, isomerization, and aromatization reactions. All these processes are catalysed by a solid acid catalyst based on alumina, silica, and zeolites. Acidic clays and mixed Al2O3 /SiO2 were originally used for this process in the 1940s but since the 1960s these catalysts have been largely superseded by zeolites. The principal zeolite used for catalytic cracking is zeolite Y, in which the extra-framework cations have been replaced with lan thanoid ions, typically a mixture of La, Ce, and Nd. The mechanism of the catalytic cracking initially involves protonation of the alkane or alkene chain by the Brønsted acid sites in the zeolite pores followed by cleavage of the C-C bond in the -position to the C atom carrying the positive charge. For example

Acidic zeolite catalysts also promote rearrangement reactions via carbonium ions. For ex ample, the isomerization of 1,3-dimethylbenzene to 1,4-dimethylbenzene probably occurs by the following steps:
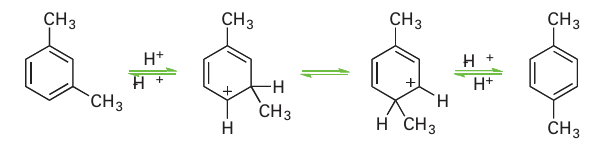
Reactions such as dimethylbenzene (xylene) isomerization and methylbenzene (toluene) disproportionation illustrate the selectivity that can be achieved with acidic zeolite catalysis. The shape selectivity of these zeolite catalysts has been attributed to a variety of processes. In reactant selectivity the molecular sieving abilities of zeolites are important as only molecules of appropriate size and shape can enter the zeolite pores and undergo a reaction. In product selectivity, a reaction product molecule that has dimensions compatible with the channels will diffuse faster, allowing it to escape; molecules that do not fit the channels diffuse slowly and, on account of their long residence in the zeolite, have ample opportunity to be converted to the more mobile isomers that can escape rapidly. A currently more favoured view of zeolite selectivity is based on transition state selectivity, where the orientation of reactive intermediates within the zeolite channels favours specific products. In the case of dimethylbenzene (xylene) isomerization the narrower intermediates formed during the generation of 1,4-dialkylbenzene molecules fit better within the pores. Another common reaction in zeolites is the alkylation of aromatics with alkenes.
Mesoporous silicates discovered in the 1990s (Sections 24.13 and 25.9) have large ordered arrays of pores in the range 1 20 nm and generate very high specific surface areas (of over 1000 m2 g 1). The large pores allow larger molecules to undergo catalytic process es, although their acidity is weak compared with the zeolites. More importantly other catalytic centres, such as nanoparticles of metals, alloys, and metal oxides such as platinum or Pt/Sn, may be deposited within the mesostructured channels. As one example, Co deposited on the mesoporous silica support MCM-416 promotes the cycloaddition of alkynes with alkenes and carbon monoxide to produce cyclopentenones in the Pauson–Khand reaction:

Other porous inorganic framework materials (Section 24.14) are also being investigated as potential catalysts, either in their own right or as hosts for active species. For example, the d-metal constituents of metal-organic frameworks can take part in redox reactions and also have very large pores that can incorporate nanoparticles of metals, such as Pt.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















