
Carbon-13 Nuclear Magnetic Resonance Spectroscopy
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 12-1-2022
12-1-2022
 2076
2076
Carbon-13 Nuclear Magnetic Resonance Spectroscopy
In recent years C13 nmr spectroscopy using C13 of natural abundance (1.1%) has become an important tool for organic structural analysis. That this did not happen sooner is because C13 has a much smaller magnetic moment than H1 and the small moment combined with the small natural abundance means that C13 is harder to detect in the nmr than H1 by a factor of 5700. This is a large difference and can be put in the proper context in the following way. Suppose two people are talking in a noisy room and one is trying to hear the other. The common request is "talk louder". If this is not possible then the request is "say it again" or "talk more slowly". Either of the latter requests amounts to an integration of signal versus noise and takes time. Improvement in signal-to-noise for a given communication is achieved as the square root of the time of communication. On the crucial time basis, C13 nmr signals require (5700)2≅30,000,000 times more time to get the same signal-to-noise ratio as in H1H1 nmr for the same number of nuclei per unit volume. This is a problem.
Electronic improvements and use of communication theory, with emphasis on the "say-it-again" technique, have provided the means for obtaining routine C13 spectra for even fairly dilute solutions of quite complex molecules.
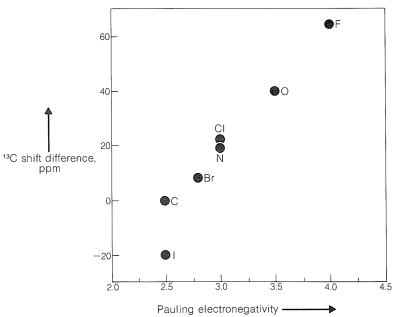
Some of the same kinds of structural effects are important for C13 chemical shifts as for proton chemical shifts (Section 9-10E). For example, there is a similar parallel between C13 shift differences in compounds of the type CH3−CH2−X and electronegativity (Figure 9-47) as between the corresponding proton shifts and electronegativity (Figure 9-28). It is important to notice that C13 shifts in ppmppm units are much larger than those of protons. This is because carbon uses pp orbitals in forming bonds, whereas hydrogen uses ss orbitals. We therefore will expect to find the the nuclei of other elements that use p orbitals in bonding, such as N15, F19, and P31, also will have larger shifts than for protons, as indeed they do.
A structural application of C13 nmr, which shows its power in an area where H1 nmr is indecisive, is shown in Figure 9-48. Here, we see the high-field C13 resonances of a substance known variously as Coumadin, or the sodium salt of warfarin, 14, which is used widely as a blood anticoagulant in the treatment of diseases such as phlebitis. It also has substantial utility as a rat poison because of its anticoagulant action.

Figure 9-48 show no splittings of the C13 resonances by the hydrogens directly attached to the carbons, even though such splittings normally are quite large (125−320Hz)). The reason is that, while the C13 spectra were taken, protons were simultaneously subjected to strong irradiation at their resonance frequency, which, as far as spin-spin splitting goes, causes them to act as nonmagnetic nuclei, such as Cl, Br, and I. This double-resonance technique for removing the C13−HC splittings is called proton decoupling.

Figure 9-48: Proton-decoupled C13 nmr spectrum at 15.1MHz of the upfield region of (a) the sodium salt of warfarin (14) showing on the right side the resonances of C11, C12, and C14. This part of the spectrum can be compared with the more complete C13 spectrum (b) of warfarin itself (16 and 17). The gaggle of evenly spaced sharp peaks toward the center of the spectrum arises from the solvent, O(CD2CD2)2O.
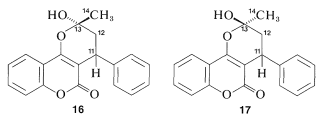
There is no indication of any abnormality in the chemical shifts of carbons 11, 12, and 14 shown in Figure 9-48a. Furthermore, there is a downfield resonance 216.5ppm from the carbons of TMS (not shown in Figure 9-48a) which is typical of a C=O carbon corresponding to C13. When 14 is treated with acid, we expect the product (warfarin) of structure 15 to be formed, which should have a C13 spectrum much like that shown in Figure 9-48a.

Figure 9-48a now come in unequal pairs. Futhermore, the C=O carbon resonance of 14 has disappeared and two new lines are observed at 99.6 ppm and 103.4 ppm farther upfield.
The C13 data indicate clearly that warfarin is not 15 in solution but is a mixture of two diastereomers (16 and 17, called cyclic hemiketals) resulting from addition of the −OH group of 15 to the C=O bond:
This is one example of the power of C13 nmr to solve subtle structural problems.
88Although the principal isotopes of Cl, Br, and I have magnetic properties, because of the special character of all of these isotopes, they act in organic compounds as though they were nonmagnetic.
99Resonance in the sense used here means that the radio-frequency absorption takes place at specified "resonance" frequencies. However, you will see that almost all of the forms of spectroscopy we discuss in this book involve "resonance" absorption in the same sense.
10 Here, γ is in Hz per gauss; physicists usually define γ in radians per second per gauss.
From the Greek prefix dia meaning through, across. The opposite of diamagnetic is paramagnetic; para meaning alongside. We shall use this term later.
In addition to giving better separation of the lines and clearer spectra, going to higher fields also has the beneficial effect of increasing the proportions of the nuclei in the +1/2 state, thereby giving more intense, easier-to-detect resonances.
Many other proton-shift values are available in NMR Spectra Catalog, Volume 1 and 2, Varian Associates, Palo Alto, Calif., 1962, 1963.
If two rings were present, this also would give four hydrogens less than the alkane. However, two rings are not possible with only three carbons.
 الاكثر قراءة في التشخيص العضوي
الاكثر قراءة في التشخيص العضوي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


