

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
How Does RNA Interference Work
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
18-6-2021
3122
How Does RNA Interference Work?
KEY CONCEPTS
- MicroRNAs regulate gene expression by base pairing with complementary sequences in target mRNAs.
- RNA interference triggers degradation or translation inhibition of mRNAs complementary to miRNA or siRNA; it can also lead to mRNA activation.
- dsRNA may cause silencing of host genes.
RISC is the complex of a microRNA bound to an Argonaute protein complex that carries out translational control, guided to its mRNA target in the cytoplasm by the associated miRNA. Two primary mechanisms are used to control mRNA expression: (1) degradation of the mRNA or (2) inhibition of translation of the mRNA. Plants use miRNA primarily for mRNA degradation, whereas animals primarily use translation inhibition. Both groups, however, do use both mechanisms. The choice is primarily determined by the degree of base pairing between the miRNA and the mRNA. The higher the degree of base pairing, the more likely that the target mRNA will be degraded, primarily through a 5′ to 3′ pathway. Whereas most examples of miRNA mechanisms are inhibitory, there are a few examples where a miRNA is required for translation activation.
This is an essential mechanism for fine-tuned control of translation in eukaryotes. As noted earlier, eukaryotic mRNA is much more stable than bacterial mRNA, and because degradation of some mRNAs is stochastic, cells must be able to tightly control which mRNAs will be translated into protein. During development, it is especially critical to ensure rapid and complete turnover of key mRNAs.
RISC uses the miRNA as a guide to scan mRNAs by sliding along the RNA looking for a small 2- to 4-nucleotide region of homology that is then extended to an 8-bp seed region in order to initiate full pairing by a stepwise mechanism. These regions are usually found in an AU-rich region in the 3′ UTR of mRNAs, with a few found in the ORF. A given mRNA may contain multiple target sites and thus respond to different miRNAs under different conditions. In binding to its target site on the mRNA, the 5′ end of the miRNA from about nucleotide 2 to 8 is the most important—the seed sequence. These should have perfect base pairing.
Once binding has occurred, several different outcomes are possible, as shown in FIGURE 1, ranging from various mechanisms of inhibiting translation to degradation of the message. RISC can interfere with translation already under way from a ribosome by blocking translation elongation (Figure 1a) or by inducing proteolysis of the nascent polypeptide being produced
(Figure 1b).

FIGURE 1. Mechanisms of miRNA-mediated gene silencing. (a) Postinitiation mechanisms. MicroRNAs (miRNAs; red) repress translation of target mRNAs by blocking translation elongation or by promoting premature dissociation of ribosomes (ribosome dropoff). (b) Cotranslational protein degradation. This model proposes that translation is not inhibited but rather that the nascent polypeptide chain is degraded cotranslationally. The putative protease is unknown. (c–e) Initiation mechanisms. MicroRNAs interfere with a very early step of translation, prior to elongation. (c) Argonaute proteins compete with eIF4E for binding to the cap structure (red dot). (d) Argonaute proteins recruit eIF6, which prevents the large ribosomal subunit from joining the small subunit. (e) Argonaute proteins prevent the formation of the closed-loop mRNA configuration by an ill-defined mechanism that includes deadenylation. (f) MicroRNA-mediated mRNA decay. MicroRNAs trigger deadenylation and subsequent decapping of the mRNA target. Proteins required for this process are shown, including components of the major deadenylase complex (CAF1, CCR4, and the NOT complex), the decapping enzyme DCP2, and several decapping activators (dark blue circles). (Note that mRNA decay could be an independent mechanism of silencing or a consequence of translational repression, irrespective of whether repression occurs at the initiation or postinitiation levels of translation.) RISC is shown as a minimal complex including an Argonaute protein (yellow) and GW182 (blue). The mRNA is represented in a closedloop configuration achieved through interactions between the cytoplasmic poly(A) binding protein (PABPC1; bound to the 3′ poly(A) tail) and eIF4G (bound to the cytoplasmic cap-binding protein eIF4E).
Reprinted from Cell, vol. 132, A. Eulalio, E. Huntzinger, and E. Izaurralde, Getting to the root
of miRNA …, pp. 9–14. Copyright 2008, with permission from Elsevier [http://www.sciencedirect.com/science/journal/00928674].
RISC can also inhibit translation initiation in multiple ways, presumably by virtue of the fact that the central domain of the Ago polypeptide has homology to the cap-binding initiation factor, eIF4E (see the Translation chapter). RISC can bind to the cap and inhibit eIF4E from joining (Figure 1c) or prevent the large 60S ribosomal subunit from joining (Figure 1d). RISC can also prevent the circularization of the mRNA by preventing cap binding to the poly(A) tail (Figure 1e). One way in which RISC can promote mRNA degradation is by promoting deadenylation and subsequent decapping of the message (Figure 1f). RISC can also indirectly facilitate mRNA degradation by targeting the mRNA to existing degradation pathways. RISC mediates the sequestering of mRNAs to processing centers called P bodies (cytoplasmic processing bodies). These are sites where mRNA can be stored for future use and where decapped mRNA is degraded .
Although translation repression is the most common outcome (based on current knowledge) for miRNA action, miRNAs can also lead to translation activation. The 3′ UTR of tumor necrosis factor- α (TNF-α) contains a regulatory RNA element called an AU-rich element, or ARE. These are common elements that are usually involved in translation repression . In this case, the ARE is involved in activation of translation of the mRNA upon serum starvation. This activation has now been shown to require RISC and its miRNA in a complex with the fragile X–related protein FXR1, an RNA-binding protein. The question of how the RISC complex is converted from its normal repression action to activation hinges on the exact makeup of the complex. Different protein partners in the complex will elicit different responses. Serum starvation leads to the recruitment of FXR1, which alters RISC action, perhaps because RISC is communicating between the 3′ UTR and the mRNA cap, where translation initiation is controlled.
One of the earliest known examples of RNAi in animals was discovered in the nematode C. elegans as the result of the interaction between the regulator gene lin4 (lineage) and its target gene, lin14. The lin14 gene produces an mRNA that regulates larval developmental timing; it is a heterochronic gene. Lin14 is a critical protein for specifying the timing of mitotic divisions in a special group of cells. Both loss-of-function mutations and gain-offunction mutations result in embryos with severe defects.
Expression of lin14 is controlled by lin4, which codes for a miRNA. The lin4 transcripts are complementary to a 10-base sequence that is imperfectly repeated seven times in the 3′ UTR of the lin14 mRNA. lin4 miRNA binds to these repeats both with a bulge (due to imperfect pairing) and without a bulge in the perfectly paired repeats and regulates expression at a posttranslation initiation step as shown in Figure1f.
As described for bacterial sRNA, a dynamic interplay can take place between different elements that modulates the ultimate outcome. Multiple mechanisms control the reaction between RISC and its target mRNA. Proteins can bind to mRNA target sequences to prevent their utilization by RISC, and the 3′ UTR of the mRNA itself may have alternate base-pairing structures that can influence the ability of RISC to identify and target a binding site. miRNA precursors can be edited by ADAR, an adenosine deaminase editing enzyme, which converts A to I and disrupts base pairing of A to U. This can result in either activation or inactivation of an
miRNA. Multiple Ago proteins allow an interesting modulation mechanism. In the plant Arabidopsis, alternate Ago proteinsbinding to one mi RNA can lead to alternate outcomes. Ago1 binds to most miRNAs and causes mRNA target degradation. Ago10, described as a decoy, can bind the same set of miRNAs as Ago1 and prevent that target degradation, as seen in FIGURE2. C. elegans and some viruses can express an ncRNA, which can interfere with Dicer and alter the mRNA profile of a cell. Even more interesting is that some genes have alternate poly(A) cleavage sites and are able to produce two versions of the mRNA, differing in the length and therefore the makeup of the 3′ UTR, to either contain more or fewer miRNA target sites.
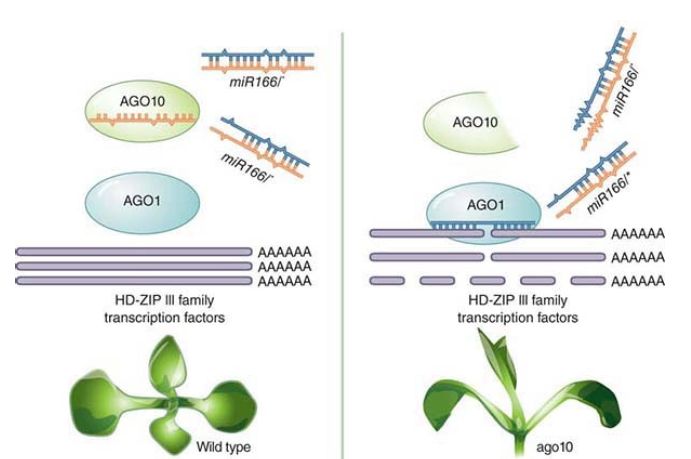
FIGURE 2. Arabidopsis AGO10 predominantly associates with miR166/165. The duplex structure of miR166/165 determines their specific association with AGO10. AGO10 competes with AGO1 for miR166/165 binding. The decoy activity of AGO10 drives shoot apical meristem development.
Modified from H. Zhu, et al. Cell 145 (2011): 242–256.
RNAi has become a powerful technique for ablating the expression of a specific target gene in invertebrates. The technique was initially more limited in mammalian cells, which have the more generalized response to dsRNA of shutting down protein synthesis and degrading mRNA. FIGURE 3 shows that this happens as a result of two reactions. The dsRNA activates the enzyme PKR, which inactivates the translation initiation factor eIF2a by phosphorylating it. It also activates 2′,5′-oligoadenylate synthetase, whose product activates RNase L, which degrades all RNAs in the cell. It turns out, however, that these reactions require dsRNA that is longer than 26 nucleotides. If shorter dsRNA (21 to 23 nucleotides) is introduced into mammalian cells, it triggers the specific degradation of complementary RNAs, just as with the RNAi technique in worms and flies.

FIGURE 3.Long dsRNA inhibits protein synthesis and triggers degradation of all mRNA in mammalian cells, as well as having sequence-specific effects.
RNA interference is related to natural processes in which gene expression is silenced. Plants and fungi show RNA silencing (sometimes called posttranscriptional gene silencing), in which
dsRNA inhibits expression of a gene. The most common sources of the RNA are a replicating virus or a transposable element. This mechanism may have evolved as a defense against these elements. When a virus infects a plant cell, the formation of dsRNA triggers the suppression of expression from the plant genome. Similarly, transposable elements also produce dsRNA. RNA silencing has the further remarkable feature that it is not limited to the cell in which the viral infection occurs: It can spread throughout the plant systemically. Presumably, the propagation of the signal involves passage of RNA or fragments of RNA. It may require some of the same features that are involved in movement of the virus itself. RNA silencing in plants involves an amplification of the signal by an RNA-dependent RNA polymerase, which uses the siRNA as a primer to synthesize more RNA on a template of complementary RNA.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















