
CpG Islands Are Regulatory Targets
 المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
 المصدر:
LEWIN’S GENES XII
المصدر:
LEWIN’S GENES XII
 الجزء والصفحة:
الجزء والصفحة:
 9-5-2021
9-5-2021
 2347
2347
CpG Islands Are Regulatory Targets
KEY CONCEPTS
- CpG islands surround the promoters of constitutively expressed genes where they are unmethylated.
- CpG islands also are found at the promoters of some tissue-regulated genes.
- The human genome has approximately 29,000 CpG islands.
- Methylation of a CpG island prevents activation of a promoter within it.
- Repression is caused by proteins that bind to methylated CpG doublets.
The origin of DNA methylation may have been as a defense mechanism to prevent inserted sequences such as viruses and transposable elements from being expressed. In both plants and animals, these sequences and simple repeat sequences are uniformly methylated.
It is now possible to examine the full methylome of an entire genome in multiple tissues at multiple times during development. The majority of methylation occurs in CpG islands in the 5′ regions of some genes and is connected with the effect of methylation on gene expression. These islands are detected by the presence of an increased density of the dinucleotide sequence CpG (CpG = 5′-CG-3′). A significant minority of methylation, however, is not found in CpG islands.
The CpG doublet occurs in vertebrate DNA at only about 20% of the frequency that would be expected from the proportion of G-C base pairs. (This may be because when CpG doublets are methylated on C, spontaneous deamination of methyl-C converts it to T, which, if incorrectly repaired, introduces a mutation that removes the doublet.) In certain regions, however, the density of CpG doublets reaches the predicted value; in fact, it is increased by a factor of 10 relative to the rest of the genome. The CpGdoublets in thes e regions are generally unmethylated.
These CpG-rich islands have an average G-C content of about 60%, compared with the 20% average in bulk DNA. They take the form of stretches of DNA typically 1 to 2 kb long. The human genome has about 45,000 such islands. Some of the islands are present in repeated Alu elements and may just be the consequence of their high G-C content. The human genome sequence confirms that, excluding these, there are about 29,000 islands. The mouse genome has fewer islands, about 15,500. About 10,000 of the predicted islands in both species appear to reside in a context of sequences that are conserved between the species, suggesting that these may be the islands with regulatory significance. The structure of chromatin in these regions has changes associated with gene expression when the CpG islands are unmethylated . The content of histone H1 is reduced (which probably means that the structure is less compact); the other histones are extensively acetylated (a feature that tends to be associated with gene expression); and DNase-hypersensitive sites or sites nearly devoid of histone octamers (as would be expected of active promoters) are present. The presence of methylated CpG sites precludes the presence of the histone variant H2A.Z in nucleosomes.
In several cases, CpG-rich islands begin just upstream of a promoter and extend downstream into the transcribed region before petering out. FIGURE 1 compares the density of CpG doublets in a “general” region of the genome with a CpG island identified from the DNA sequence. The CpG island surrounds the 5′ region of the APRT gene, which is constitutively expressed.
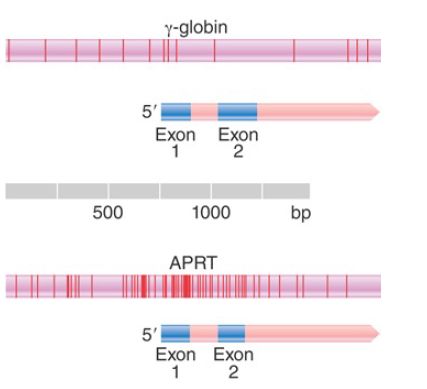
FIGURE 1. The typical density of CpG doublets in mammalian DNA is ~1/100 bp, as seen for a γ-globin gene. In a CpG-rich island, the density is increased to more than 10 doublets/100 bp. The island in the APRT gene starts ~100 bp upstream of the promoter and extends ~400 bp into the gene. Each vertical line represents a CpG doublet.
All of the housekeeping genes that are constitutively expressed have CpG islands; this accounts for about half of the islands. The remaining islands occur at the promoters of tissue-regulated genes; approximately 50% of these genes have islands. In these cases, the islands are unmethylated irrespective of the state of expression of the gene, so that CpG island methylation is not correlated with transcriptional state for tissue-specific genes. The presence of unmethylated CpG-rich islands may be necessary, but is not sufficient, for transcription. Thus, the presence of unmethylated CpG islands may be taken as an indication that a gene is potentially active rather than inevitably transcribed. Many islands that are unmethylated in an animal become methylated in cell lines in tissue culture (or in some cancers); this could be connected with the inability of these lines to express all of the functions typical of the tissue from which they were derived. The one clear example in which there is a strong correlation between promoter methylationand gene expression is w hen promoter CpG islands become methylated in the mammalian inactive X chromosome .
Methylation of a CpG island can affect transcription. One of two mechanisms can be involved:
Methylation of a binding site for some factor may prevent it from binding. This happens in a case of binding to a regulatory site other than the promoter .
Methylation may cause specific repressors to bind to the DNA. Repression is caused by either of two types of protein that bind to methylated CpG sequences. The protein MeCP1 requires the presence of several methyl groups to bind to DNA, whereas MeCP2 and a family of related proteins can bind to a single methylated CpG base pair. This explains why a methylation-free zone is required for initiation of transcription. Binding of proteins of either type prevents transcription in vitro by a nuclear extract.
MeCP2, which directly represses transcription by interacting with complexes at the promoter, also interacts with the Sin3 repressor complex, which contains histone deacetylase activities. This observation provides a direct connection between two types of repressive modifications: methylation of DNA and deacetylation of histones. Although promoters that contain CpG islands (approximately 60% CpG density) or that show no CpG enrichment (approximately 20% CpG density) exhibit a generally poor correlation between promoter methylation and transcription, a third class of promoters appears to be consistently regulated by CpG methylation. Approximately 12% of human genes contain so-called weak CpG islands, in which the density of CpGs is about 30%, intermediate between the other two classes of promoters. These genes show a strong inverse relationship between promoter CpG methylation and RNA polymerase II occupancy.
The absence of methyl groups is associated with gene expression (or at least the potential for expression). However, supposing that the state of methylation provides a general means for controlling gene expression presents some difficulties. In the case of Drosophila melanogaster (and other Dipteran insects), there is very little methylation of DNA (although one methyltransferase, Dnmt2, has been identified, its importance is unclear), and there is no methylation of DNA in the nematode Caenorhabditis elegans or in yeast. The other differences between inactive and active chromatin appear to be the same as in species that display methylation. Thus, in these organisms, any role that methylation has in vertebrates is replaced by some other mechanism.
The three changes that occur in typical active genes are as follows:
- A hypersensitive chromatin site(s) is established near the promoter.
- The chromatin of a domain, including the transcribed region, becomes more sensitive to DNase I.
- The DNA of the same region is undermethylated.
All of these changes are necessary for transcription.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


