
Solids, Liquids, and Gases: A Molecular Comparison
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 28-4-2020
28-4-2020
 1418
1418
Solids, Liquids, and Gases: A Molecular Comparison
The physical properties of a substance depends upon its physical state. Water vapor, liquid water and ice all have the same chemical properties, but their physical properties are considerably different. In general covalent bonds determine: molecular shape, bond energies, chemical properties, while intermolecular forces (non-covalent bonds) influence the physical properties of liquids and solids. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. A similar model can be applied to liquids, but it must take into account the nonzero volumes of particles and the presence of strong intermolecular attractive forces.

Figure 1.1 : The three common states of matter. From the left, they are solid, liquid, and gas, represented by an ice sculpture, a drop of water, and the air around clouds, respectively. .
The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the intermolecular forces. The kinetic energy keeps the molecules apart and moving around, and is a function of the temperature of the substance. The intermolecular forces are attractive forces that try to draw the particles together (Figure 1.2
). A discussed previously, gasses are very sensitive to temperatures and pressure. However, these also affect liquids and solids too. Heating and cooling can change the kinetic energy of the particles in a substance, and so, we can change the physical state of a substance by heating or cooling it. Increasing the pressure on a substance forces the molecules closer together, which increases the strength of intermolecular forces.
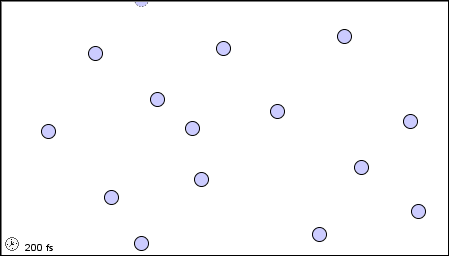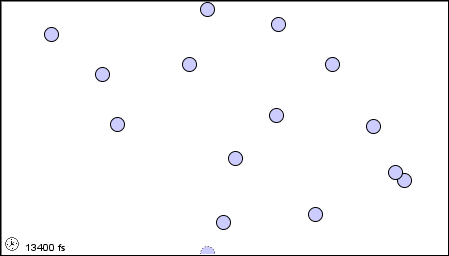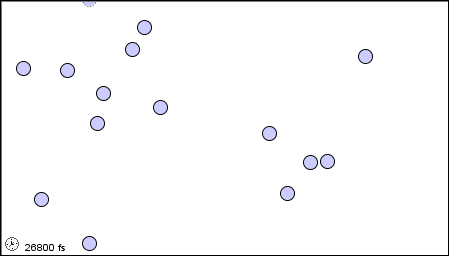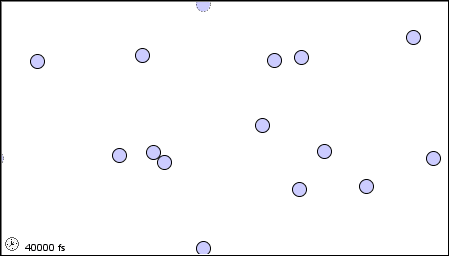 |
 |
 |
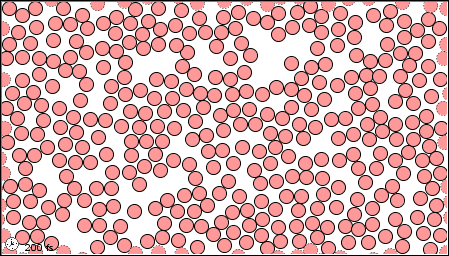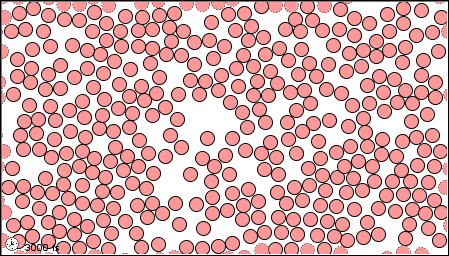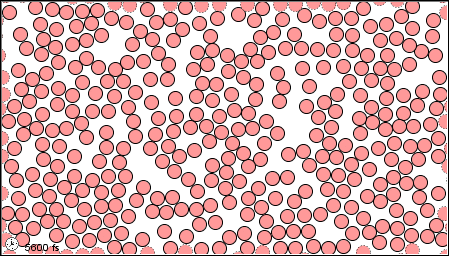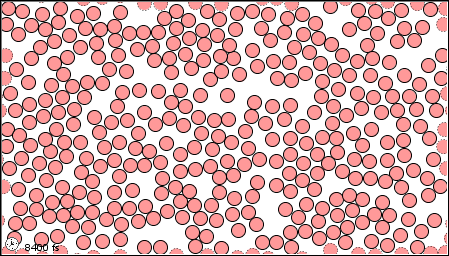
| (a) in the gaseous state |
(b) as a liquid |
(c) in solid form |
Figure 1.2 : Molecular level picture of gases, liquids and solids.
Properties of Gases
- A collection of widely separated molecules
- The kinetic energy of the molecules is greater than any attractive forces between the molecules
- The lack of any significant attractive force between molecules allows a gas to expand to fill its container
- If attractive forces become large enough, then the gases exhibit non-ideal behavior
Properties of Liquids
- The intermolecular attractive forces are strong enough to hold molecules close together
- Liquids are more dense and less compressible than gasses
- Liquids have a definite volume, independent of the size and shape of their container
- The attractive forces are not strong enough, however, to keep neighboring molecules in a fixed position and molecules are free to move past or slide over one another
Thus, liquids can be poured and assume the shape of their containers.
Properties of Solids
- The intermolecular forces between neighboring molecules are strong enough to keep them locked in position
- Solids (like liquids) are not very compressible due to the lack of space between molecules
- If the molecules in a solid adopt a highly ordered packing arrangement, the structures are said to be crystalline
Due to the strong intermolecular forces between neighboring molecules, solids are rigid.
- Cooling a gas may change the state to a liquid
- Cooling a liquid may change the state to a solid
- Increasing the pressure on a gas may change the state to a liquid
- Increasing the pressure on a liquid may change the state to a solid
 الاكثر قراءة في اخرى
الاكثر قراءة في اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


