
Molecularity and Order are Identical for Elementary Reactions or Steps
 المؤلف:
Arun Bahl, B.S. Bahl and G.D.Tuli
المؤلف:
Arun Bahl, B.S. Bahl and G.D.Tuli
 المصدر:
Essential Physical Chemistry
المصدر:
Essential Physical Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 23-9-2018
23-9-2018
 823
823
Molecularity and Order are Identical for Elementary Reactions or Steps
The rate of an elementary reaction is proportional to the number of collisions between molecules (or atoms) of reactions. The number of collisions in turn is proportional to the concentration of each reactant molecule (or atom). Thus for a reaction.
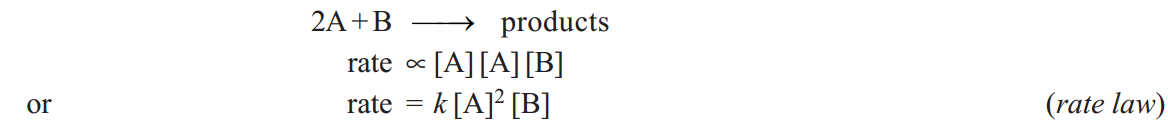
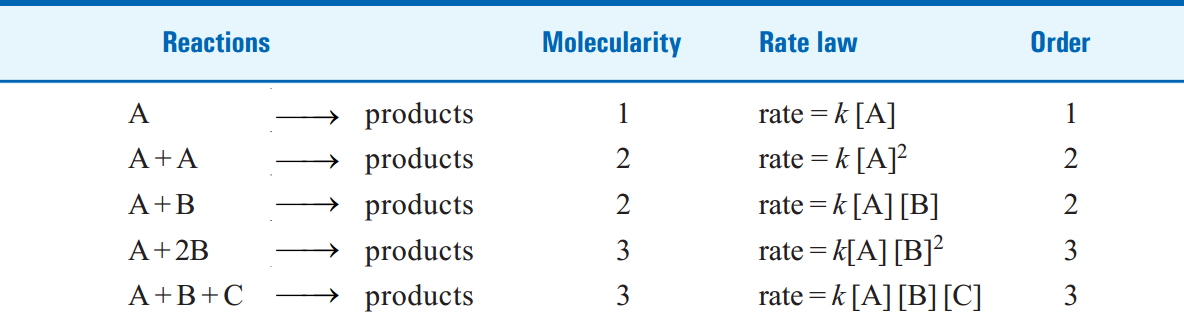
Two molecules of A and one molecule of B are participating in the reaction and, therefore, molecularity of the reaction is 2 + 1 = 3. The sum of powers in the rate law is 2 + 1 and hence the reaction order is also 3. Thus the molecularity and order for an elementary reaction are equal.
TABLE 1.1. MOLECULARITY AND ORDER FOR ELEMENTARY REACTIONS.
 الاكثر قراءة في حركية التفاعلات الكيميائية
الاكثر قراءة في حركية التفاعلات الكيميائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


