


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 10-11-2015
Date: 10-11-2015
Date: 27-10-2015
|
Prenatal Diagnosis
INTRODUCTION
Genetic and environmental factors have an influence on various stages of development in the zygote, the preembryo, embryo, the fetus and the neonate. With the growth of genetic technology and development of high resolution ultrasound in recent years, it has become possible to detect more than 5,000 defects of hereditary and non-hereditary origin in the prenatal period. Prenatal diagnosis focuses on the diagnosis of various birth defects. Prior to development of this technology couples at risk were left with options of a risk of genetic disease or choosing other reproductive options like contraception, sterilization, or adoption. Today, these at-risk couples can make an informed choice about continuation or termination of pregnancy if a serious abnormality is detected, or think about early effective management to improve quality of life for their child. Another advantage of prenatal diagnosis is, that with normal test results, at risk couple is reassured.
Various invasive and non-invasive techniques are now available for prenatal diagnosis. The current commonly used and reliable methods of prenatal diagnosis are ultrasound, amniocentesis and chorionic villus biopsy. Maternal serum screening test at 14-16 weeks is added to these tests to pick up high-risk pregnancies for Down syndrome, and other trisomies and neural tube defects. Ultrasound is the most important tool in the detection of morphological defects of the fetus and for needle guidance in interventional techniques. For defects of genetic origin fetal tissue sampling is necessary. These tissues are used for diagnosis of chromosomal, metabolic and molecular genetic diseases.
INDICATIONS FOR PRENATAL DIAGNOSIS
Advanced Maternal Age
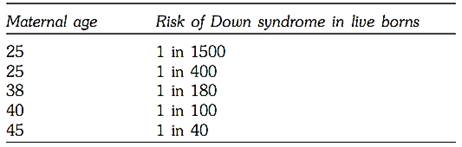
The most common indication for prenatal diagnosis is advanced maternal age. There is sufficient data to prove that there is an increased association between advanced maternal age and Down syndrome (Table.1) although other autosomal trisomies are also reported. The cause of chromosomal error in advanced maternal age is attributed to aging of the egg, which is believed to be due to the ovum being in suspended prophase. The average advanced maternal age is considered as 35 at the time of delivery, and most women at this age are offered amniocentesis or chorionic villous sampling.
Table 1: Co-relation between maternal age and Down syndrome
Previous Child with a Chromosomal Disorder
A couple with a history of a chromosomal disorder in a previous child, are at an increased recurrence risk, for chromosomal disorder. The risk is increased if either of the partners have balanced chromosomal rearrangement .The risk generally varies between 2 to 15%, except in a G:G translocation, where it is 100%.
Family History of a Chromosomal Disorder
In such cases, the need for prenatal diagnosis will depend on the type of chromosomal rearrangement in an index case. The karyotype of such a couple determines their recurrence risk, and prenatal diagnosis can follow.
History of Single Gene Disorder in a Previous Child or Family
Prenatal diagnoses of many single gene disorders of biochemical or molecular origin is diagnosed in pregnancy and are discussed in chapters focused on these. To plan a precise prenatal test laboratory diagnosis of an index case is important in most cases. Physicians caring for children with birth defects must make every attempt to establish a diagnosis in an index case to provide active management of the affected child, and for prenatal planning of future pregnancies.
A Positive Triple Test
The triple test is based on the estimation in the maternal serum of certain biochemical markers present in pregnancy. These are serum alfafetoprotein beta hcg, and serum estriol. (AFP, PhCG and uE3). Low AFP and high beta hCG and low estriol are indicative of increased risk of fetal aneuploidy, specially Down syndrome. With a positive triple test, fetal chromosomal studies are indicated. High alfafetprotein levels are indicative of an open neural tube defect, although certain gastrointestinal tract anomalies also are associated with it. A good ultrasound anomaly scan is important to confirm an anomaly.
Abnormal Ultrasound Findings (Soft markers for chromosomal syndromes)
Many chromosomal abnormalities, besides leading to mental retardation, have morphological abnormalities. Some of these are gross and can be picked up on ultrasound easily, while others are called soft markers. Confirmation of a syndrome in the affected pregnancy is of immense value in ascertaining a cause, deciding the time and mode of delivery, and therapy whenever possible. Accurate genetic counseling can be offered on a confirmed diagnosis.
Infertility
Many infertile couples on assisted reproductive programs have chromosomal rearrangements as a cause of their infertility. Once pregnancy is achieved, they maybe at an increased risk for a birth defect. Prenatal diagnosis can help these couples ensure an ongoing healthy pregnancy, or if abnormal, they can make an informed choice/decision.
History of Neural Tube Defects in the Previous Child or Family
Neural tube defects are mainly inherited as multifactorial disorders. When chromosomal factors are ruled out, couples with such a history should be offered maternal serum AFP screening and a first and second trimester ultrasound scan. A 95% pick up rate can be expected in such cases.
Maternal Illness, Maternal Genetic Disease and Bad Obstetric History
With improved neonatal and pediatric care, men and women with genetic disorders can reach adulthood and reproduce. In such parents if desired, prenatal tests can be planned.
Pregnant mothers with poorly controlled insulin dependent diabetes, or epilepsy are at risk for fetal structural defects either due to their disease, or due to the drugs used to treat it. Mothers should be counseled accordingly and should be offered fetal ultrasound for detection of possible anomalies. Women with history of repeated fetal loss due to chromosomal defects in the products of conception should be offered prenatal cytogenetic evaluation.
TECHNIQUES INVOLVED IN PRENATAL DIAGNOSIS
Current techniques involved prenatal diagnosis are divided into following groups:
Other Techniques
COUNSELING AND INFORMED CONSENT
For successful prenatal diagnosis, the couple needs to be counseled. Counseling should elicit a detailed clinical and family history. The counselor should confirm the indication for requested diagnosis with reference to the detection of the abnormality in question. The patient should be made aware that the laboratory test is performed as per the indication only. The safety and efficacy of the obstetric and laboratory tests should be explained. The couple should be informed about the possibility of the need for a second sample, which may be required in case of culture failure or ambiguous results.
Informed written consent is important and should be obtained from all the patients before the procedures are undertaken.
In India, prenatal diagnosis is allowed only under a specified Act. Any physician or a geneticist who provides prenatal diagnostic services in the form of counseling, obstetric procedures of fetal tissue sampling or the laboratory testing is required to obtain a license from the respective state Government, As per the act. The Pre-natal Diagnostic Techniques (Regulation and Prevention of Misuse) Act, 1994. Amended as the Preconception and Prenatal Diagnostic Techniques (Prohibition of Sex Selection ACT 2003).
Procedures
Non-invasive Techniques
The Triple Test
It was observed in the West that in spite of offering prenatal cytogenetic studies to all women of advanced maternal age, the incidence of Down syndrome was not lowered. This is because many women at a much younger age also give birth to Down syndrome babies, but are not offered the triple test and are therefore not screened.
At 16 week’s gestation mothers carrying Down’s babies have an alteration in the levels of certain biochemical markers as compared with normal pregnancies of the same gestational age. These markers are AFP beta hCG, and estriol (Table 2). The markers are also predictive of risk for other fetal aneuploidy.
Mothers with a positive screen test are considered as high- risk for fetal chromosomal aneuploidy and should be offered amniocentesis. Factors which can affect the values are maternal age, maternal weight, diabetes and gestational age and should be taken into account. Median of the multiple values (MOM) is calculated for interpretation of the results.
Table 2: Maternal serum triple test risk estimates

Obstetric Ultrasound
Ultrasound is a valuable non-invasive tool of prenatal diagnosis, and is used for detection of structural anomalies of the fetus as well as for needle guidance in various invasive procedures.
Fetal organ development is normally completed by 18 weeks gestation. With high resolution ultrasound it has become possible to look at most of the developmental defects of the fetus, like neural tube defects, cardiac anomalies or skeletal malformations. Growth of the fetus continues till term, hence follow up scan for head size, limb measurement and renal function needs to be considered.
Many chromosomal defects have some ultrasound markers, and usually are called as soft markers (Fig. 1 and Table.3). Once such markers are observed, amniocentesis or fetal blood sampling should be considered for confirmation of a chromosomal syndrome.
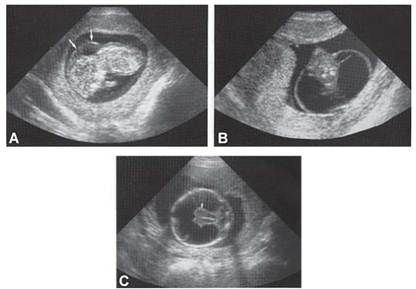
Figs.1A to C: Some ultrasound markers of chromosomal disease (A) Nuchal translucency in trisomy 21,45X, (B) cystic hygroma in 45X, (C) holoprosencephaly
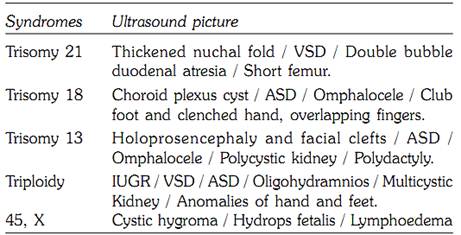
Table 3: Major chromosomal syndromes identifiable by ultrasound
Laboratory Techniques
Laboratory techniques involved in prenatal diagnosis are based on the nature of the underlying or expected defect. Basically three types of the tests are involved. These are cytogenetic (chromosomal), biochemical (enzyme assays), or molecular (DNA diagnostic). The aim of a laboratory diagnosis should be to provide rapid and reliable results.
FETAL CHROMOSOMAL STUDIES
Fetal chromosomal studies are indicated when there is an increased risk of aneuploidy on the basis of maternal age, positive triple test, previous child with chromosomal anomalies, pregnancies where one parent has a balanced structural chromosomal rearrangement, or in order to confirm fetal sex in X-linked conditions.
One of the most promising developments is interphase cytogenetics using in situ-hybridization (FISH) with non-radioactive labeled probes. The results are available in 3 days. The reliability and sensitivity of the approach is being investigated in clinical practice. Karyotyping with cell culture is still recommended to confirm the finding.
Fetal enzyme assays
Prenatal diagnosis is now possible for more than 90 inborn errors of metabolism and is indicated in all high-risk pregnancies. The amniotic fluid cells need to be grown in culture for 4-6 weeks in order to provide sufficient cells for the assay of appropriate enzymes. Currently cells from chorionic villi, direct or cultured are found to give the same results and are used when the enzymes being tested for are known to be expressed in the first trimester.
FETAL DNA DIAGNOSIS
Earlier DNA used to be extracted from amniotic cells after 3-4 weeks of culture and diagnosis was by direct demonstration of the molecular defect or by restriction fragment length polymorphisms (RFLP). Now it is possible to get sufficient amount of DNA from chorionic villi without prior culture and the test is performed earlier (9-11 weeks gestation). Chorionic villus sampling is now a preferred technique for obtaining fetal tissue for DNA analysis.
Accurate laboratory diagnosis is very important as the decision of continuation or termination of pregnancy is based on the results. Delay in results adds to anxiety leading to emotional strain. The aim of a geneticist therefore should be to provide rapid, reliable reports with a safe obstetric procedure.
FETAL TISSUE SAMPLING
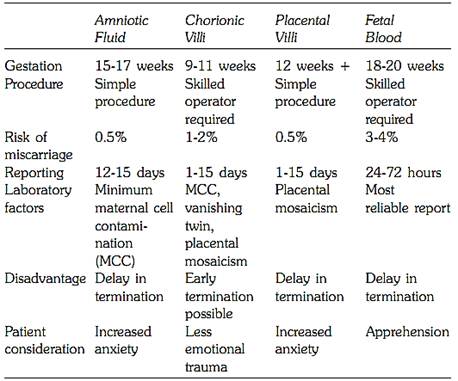
Three types of tissues are predominantly used for prenatal diagnosis. The procedures are out patient, and relatively safe in expert hands. The patient has minimum discomfort with almost no complications. One of the following three techniques is commonly used for fetal tissue sampling (Table.4).
1- Chorionic Villous sampling (CVS) (10-12 weeks) and Late CVS (12 weeks onwards)
Table 4: Procedures of fetal tissue sampling
2- Amniocentesis (15-17 weeks)
3- Fetal Blood Sampling (18-20 weeks)
In case of abnormal ultrasound findings, the physician can perform amniotic fluid and cord blood sampling during the second or third trimester. Similarly, late CVS or placental biopsy is possible in the second or third trimester. Placental biopsy is useful in cases of reduced amniotic fluid volume or abnormal ultrasound findings.
Amniotic Fluid Studies
Amniocentesis was the first technique introduced in prenatal diagnosis. The use of amniocentesis for genetic diagnosis was started in 1950, when fetal sex was determined by X- chromatin studies of amniotic fluid cells. Amniotic fluid cells were first cultured in 1966 to obtain a chromosomal pattern of the fetus. In 1967, the first prenatal diagnosis of Down’s syndrome (a balanced D/D translocation) was made.
With reliable reports and an ultrasound-guided procedure, amniocentesis has become a safe procedure. It has a low risk (0.5 to 1%) of miscarriage, thus has become integral part of modern obstetric care.
Amniocentesis is an outpatient procedure and is ideally performed between 15-17 menstrual week (Fig. 2). At this time, the ratio of viable to nonviable cells is highest. A complete ultrasound examination of the gravid uterus is made for number of fetuses and fetal viability, estimation of fetal weight by fetal biometry and placental localization. An anomaly scan for fetal malformations is done at this stage. Amniocentesis can also be done as early as 11-14 weeks (early amniocentesis), however this is not used routinely as there is an increased risk of miscarriage, and poor culture yield is likely. Amniotic fluid can be aspirated even at a later period but risk of culture failure is more likely as the ratio of viable to nonviable cells is greatly reduced after 21 weeks.
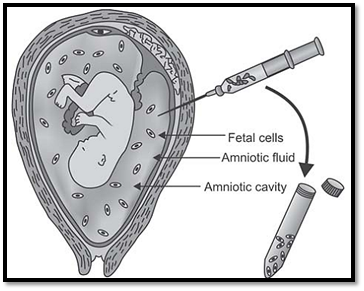
Fig. 2: Amniocentesis
Chorionic Villus sampling
First trimester fetal tissue analysis by chorionic villi has been used routinely for the last 10 years. The greatest advantage of the technique is that a genetic diagnosis is possible in the first trimester.
In 1972, spontaneous divisions were first utilized for studying chromosome preparations. In 1986, first trimester fetal karyotyping from chorionic villi (9-11 weeks) was carried out. The cells from the chorionic villi were also successfully used for biochemical and DNA analysis.
The chorion consists of an outer layer of trophoblast, and an inner layer of syncitiotrophoblast with a mesenchymal core containing blood vessels. The chorion frondosum or the future placenta is selected for aspiration. This relatively simple obstetric technique is done under ultrasound guidance as an outpatient procedure. An ideal time for chorion villus sampling is 9-11 weeks and is done by the abdominal or the transcervical route. Procedure prior to 9 weeks gestation is not recommended, as up to 8 weeks gestation, there is a phase of rapid embryonic development and organogenesis. Any intervention at this stage should be strictly avoided. 15-20 mgs of fetal material can be easily obtained for analysis from a single aspiration and is sufficient for diagnosis. Cytogenetic analysis from direct cultures is available as early as 24 hours and cultured tissues take up to two weeks for the results. In experienced hands the procedure has only a 1-2% risk of fetal loss. The only associated complication of chorionic villus sampling reported was limb reduction defects.
Advantages of Chorionic Villus Sampling
Fetal tissue sampling in multiple gestations
Amniotic Fluid Aspiration
Cytogenetic analysis is based entirely on the samples received in the laboratory. In case of multiple gestations, it should be ensured that samples are precisely obtained from individual sacs or placentae. This is possible with good ultrasound monitoring.
Chorionic Villus Sampling (CVS) in Multiple Gestations
The incidence of CVS in multiple pregnancies is on the rise due to in vitro fertilization resulting in multiple pregnancies. Often such patients need prenatal diagnosis due to advanced maternal age or other genetic reasons. It is also challenging for an obstetrician if fetal reduction of an abnormal fetus is required (Figs.3A and B).
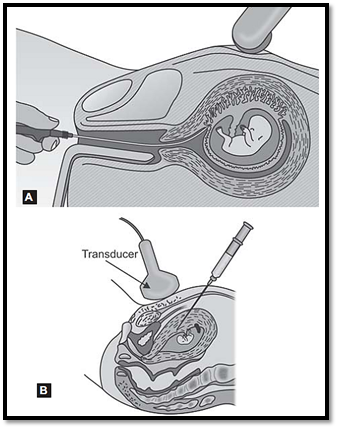
Figs .3A and B: Chorionic villous sampling. (A) Transcervical route, (B) Transabdominal route
In monozygotic twins, laboratory results will show the same genotype for both the twins. In dizygotic or multiple pregnancies, ultrasound examination must be concentrated on locating the septa and placentae. Separate devices are used for sampling from each sac to avoid contamination.
Fetal Blood Sampling (FBS)
Fetal blood sampling is the preferred test for rapid fetal karyotyping in advanced pregnancies or for confirming mosaicism observed in amniotic fluid cells or chorionic villi. Fetal nucleated blood cells can be cultured similar to shortterm lymphocyte cultures for 24-72 hours. FBS is also used for evaluation of fetal hematological disorders, DNA diagnosis and treatment of fetal anemia by transfusion. This out patient procedure is carried out at 18 -20 weeks gestation, or later under ultrasound guidance. Fetal viability, placental localization and umbilical cord insertion is confirmed before sampling (Fig. 4).
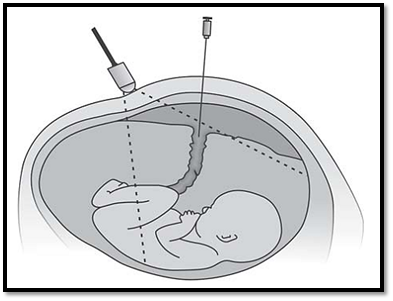
Fig. 4: Foetal blood sampling (Cordocentesis)
1-2 ml of fetal blood is aspirated. The sample is confirmed for its source, maternal or fetal. 0.5 to 2 ml of fetal blood is sufficient for cytogenetic diagnosis. There is a 3% fetal loss risk in the procedure. Maternal complications rare, but amnionitis and transplacental hemorrhage have been reported.
Fetoscopy, Fetal Skin Biopsy and Fetal Liver Biopsy
Fetoscopy is the endoscopic visualization of the fetus. The optimal time for this procedure is 18-20 weeks gestation. Because of the size of the instruments, only a limited field of view is possible and entire fetal visualization is not practicable. The procedure carries a fetal loss rate of about 3%. Some serious skin disorders can be diagnosed by a fetal skin biopsy taken via fetoscope. For some metabolic disorders, a fetal liver biopsy maybe necessary for diagnosis.
NEWER TECHNIQUES
Preimplantation Genetic Diagnosis (PGD)
Preimplantation genetic diagnosis is an extension of prenatal diagnosis, and the field emerged after the increasing success of in vitro fertilization techniques. The advantage of preimplantation diagnosis for the patient, is to have her pregnancy screened before implantation with no physical or mental stress of termination in case of abnormal results.
The first sexing of the human embryo (for an X-linked disease) was done in 1990. This was of immense value, as there are about 200 sex-linked disorders where prenatal sex determination would be useful. In the initial diagnosis, the Y specific sequence was amplified by using Polymerase Chain Reaction (PCR). Following this, in 1992 the first successful preimplantation genetic diagnosis of cystic fibrosis and Tay Sach’s disease was made.
With the use of fluorescent in situ hybridization (FISH), the diagnosis of the most common fetal aneuploidies (X, Y 13, 18, 21 and 16) can be carried out. The technique involves IVF procedures even in fertile patients, as several embryos are required to be screened. In addition it is recommended that PGD be followed by postimplantation genetic diagnosis.
Problems in Preimplantation Chromosomal Diagnosis
Techniques of Biopsy
Polar Body Biopsy
In this technique the polar body is used for analysis. It is thus non-invasive for the embryo. This method is ideal for screening aneuploidy, as a large majority of trisomies occur during the 1st Meiotic division. However, trisomy 18 occurs after meiosis 11 and therefore a 2nd Polar body analysis may be required. Only maternal defects can be analyzed by this technique and the an error in diagnosis is at times higher, due to frequent crossing over.
Cleavage Stage Biopsy
This is carried out at (6-8 Cells) on post insemination Day 3 Removal of 2 cells is possible (as single cell analysis can miss a mosaic embryo).
Blastocyst Biopsy
Multiple cells are available for analysis, resulting in a reliable diagnosis. A 1% error is possible due to confined placental mosaicism (Fig. 5).
Uterine Lavage
The embryo floats freely before implantation, and flushing is possible. However, all embryos may not be obtained.
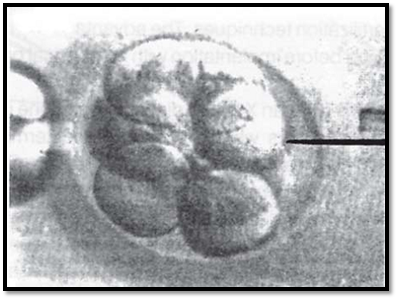
Fig. 5: Blastocyst biopsy for preimplantation genetic diagnosis
Fetal Cells in Maternal Blood
The possibility of recovering fetal cells from maternal blood was raised initially when XY metaphases in were demonstrated in maternal blood of pregnant women carrying a male fetus. In another technique, maternal blood was subjected to antibodies against paternal HLA alleles not present in the mother. Following flow sorting, fetal cells could be separated from maternal cells. Since then, newer techniques have demonstrated that fetal cells or at least fetal DNA exists in the maternal DNA. PCR for Y sequences on unsorted blood from pregnant women showed that women carrying a male fetus are far more likely to show a hybridization signal than those carrying a female fetus. A diagnosis of a fetus with trisomy 21, using flow sorted fetal erythroblasts obtained from maternal blood by using the FISH technique has been recently reported.
Problems in prenatal diagnosis
Problems in prenatal diagnosis can arise with inadequate sample, failure in culture growth, or during interpretation of results in abnormal findings. The first two problems can be resolved by a repeat sample. Interpretation of the results is the most crucial phase in prenatal diagnosis, as pregnancy management and recurrence risk estimation is entirely based on various classical or non-classical chromosomal analyses in prenatal sample.
Recommendations in Abnormal Prenatal Cytogenetic Results
Maternal Cell Contamination
In chorionic villus cytogenetic preparations, maternal cell contamination and mosaicism is known to occur more commonly than in amniotic fluid and fetal blood sampling. When such a finding is observed it is important to assess whether the results indicate a true chromosomal abnormality in the fetus, or whether this is a confined placental abnormality with a normal fetus.
Maternal cell contamination is lowest in direct preparations and short-term cultures. In long-term cultures, it can be as high as 10-14% but with proper selection of tissue and more experience in cleaning the maternal decidua, this has been reduced. Known confined placental abnormalities are 45X, trisomy 22 and trisomy 16. The latter two can lead to placental insufficiency and IUGR. However if the chromosomal abnormality is 45X, this can be either of placental origin or the fetus may have Turner’s syndrome, in which case, confirmation with amniotic fluid studies is recommended.
Mosaicism
Mosaicism can result in a major chromosome abnormality, where two or more cell lines with different karyotypes are present. True chromosomal mosaicism is one where different cell lines have originated during early post zygotic development, and are seen in the fetus. Major chromosomal trisomies, sex chromosome anomalies, chromosomal rearrangement and polyploidies can occur in the mosaic form. Post zygotic non-dysjunction is restricted to the trophoblast and extra embryonic membranes. Contamination with maternal tissue will show a mosaic cell line for fetal and maternal cells and is called confined placental mosaicism. Mosaicism can be resolved by short and long-term cultures. Mosaicism in recognized syndromes needs careful follow up by amniotic fluid studies or fetal blood sampling.
Vanishing Twin
About 7.6% pregnancies are conceived as twin pregnancies of which 6% vanish leading to one healthy twin and the other with an empty sac or remnant of the tissue. If the pregnancy is not scanned early after the missed period, the presence of one of the twins, which is going to vanish can be missed. In such a pregnancy if prenatal diagnosis is carried out by chorionic villus sampling, one can get a mixed cell line, one of which will be from persistence of trophoblast of the vanished twin, and the other from the trophoblast of the existing fetus. On some occasions, the sample may have been obtained only from the persistent trophoblast of the vanished twin. In vanishing twin cases where one fetus is healthy and the second one shows only an empty sac, patient needs to be counseled for the situation as well the need for follow up explained.
Autosomal Trisomies
Autosomal trisomies are divided into two groups. Group one, where they are associated with a clinically significant syndrome seen postnatally (Figs.6 and.7). They have a severs impact on the physical and mental development of a child, for example Trisomy 13, 18, 21. The clinical features of these syndromes are described in the chapter on chromosomal syndromes.
Group two trisomies are the ones where there is a high risk of Pseudomosaicism. In this, mosaicism is restricted only to the trophoblast and extra embryonic cells and it is not present in the fetus. Such trisomies are usually seen in chromosome number 2, 3,14, 15, 16, 20 and 22.
Trisomies of chromosome 3, 14, 16 and 22 are of placental origin hence seen more often in CVS samples, while trisomies for chromosome 2 and 20 occur frequently in amniotic fluid cultures. A fetus with trisomy 20
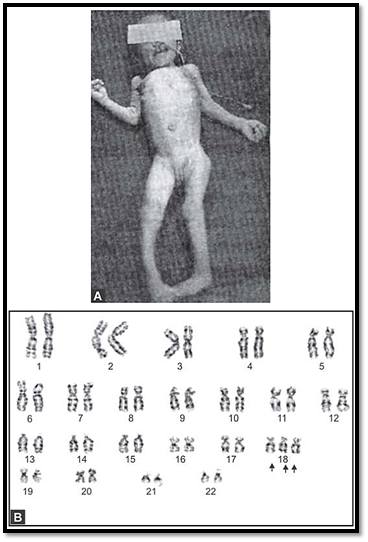
Figs 6A and B: (A) Fetus with trisomy 18. (B) Karyotype of the same fetus showing trisomy 18
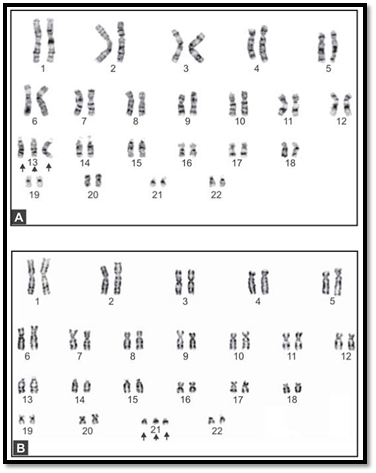
Figs. 7A and B: Prenatal karyotypes generated by culture of amniotic fluid. (A) Trisomy 21, (B) trisomy 13
mosaicism may have a normal phenotype. Trisomy for chromosome 20 may be detected in specific fetal tissues such as kidney, rectum, oesophagus and placenta. It suggests that Trisomy 20 is confined to specific fetal tissues.
Trisomy in the Clinically Recognized Syndromes
Trisomies in the clinically recognized syndromes can also present as mosaicism, and the risk to the fetus is high. Pseudomosaicism has been demonstrated in trisomy 13, 18 and mosaic trisomies of chromosomes 7, 8, 9, 13, 18, 21. Follow up by amniocentesis or fetal blood sampling is recommended in such cases (Figs 8A to E).
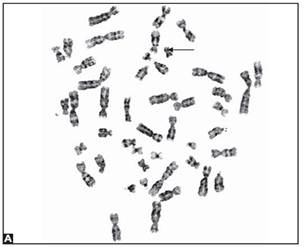
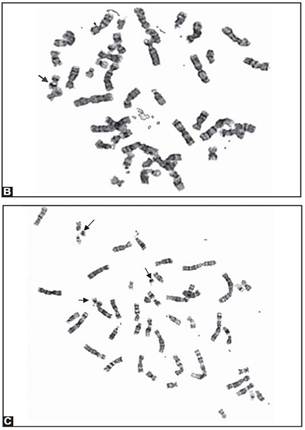
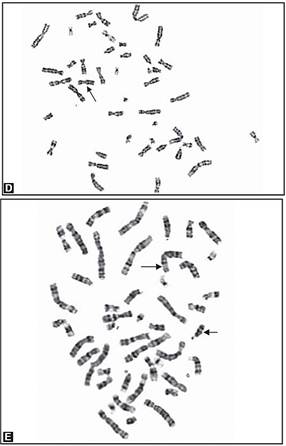
Figs 8A to E: Chromosomal abnormalities observed in fetal samples (A) Inversion Y (cord blood), (B) translocation t (14;21) cord blood, (C) trisomy21-(amniotic fluid) (D) 45XO-(chorionic villous sampling), (E) translocation t 8;15-(chorionic villous sampling)
Sex Chromosome Mosaicism
Sex chromosomal trisomies are compatible with life and symptoms vary from mild to severe hypogonadism. The clinical features are described in chromosomal syndromes. They can be seen in mosaic forms.
True mosaicism for sex chromosomes exists in patients with sex chromosome abnormalities, leading to abnormalities of genitalia or secondary sexual development. In some CVS samples this can occur due to maternal cell contamination. It is recommended that the finding be confirmed by amniotic fluid culture or fetal blood sampling. The common sex chromosome abnormalities seen are intersex states, pseudohermaphroditism or Turner’s syndrome. A brief summary of these is included in the chapter on chromosomal syndromes.
Chromosome Rearrangement
0.5% of population is known to have a chromosomal rearrangement, where the total genetic material is normal but rearranged. Types of rearrangements include translocations, pericentric and paracentric inversions and ring chromosomes. Unfortunately, phenotypes may vary from normal to severely handicapped. Hence prognosis in each individual case varies, and careful consideration is required while counseling the individual.
If such a karyotype is observed in fetal tissue, following steps are recommended:
1- Confirm parental karyotype and check for familial origin of rearrangement (maternal or paternal origin). If similar pattern is seen, and parents are normal, the risk to the fetus is low.
2- Literature survey for mental retardation or dysmorphology syndromes for correlation with the chromosome rearrangement.
3- Confirmation of the rearrangement with amniocentesis or fetal blood sampling using high-resolution banding or molecular cytogenetic techniques.
Supernumerary or Marker Chromosome
In supernumerary or marker chromosomes, assessing the prognosis is difficult. This will depend on the size of the marker chromosomes and heterochromatin involvement. The consequences can be mild to serious. Fluorescent in situ hybridization can be used to identify the segment involved. When a marker chromosome is observed, the following points need to be considered:
1- Is the marker de novo or of familial origin
2- The percentage of cells with marker chromosomes in the fetus and the parents
3- The relative size of the chromosome compared to the ‘G’ group of chromosomes
4- Confirmation of composition by AgNOR staining Literature survey for risks arising from the presence of
a marker chromosome.
Polyploidy
Postzygotic error can lead to diploid/triploid mosaicism and is seen in the vanishing twin syndrome. Triploidy is seen commonly in 1st trimester abortions and in pregnancy up to the second trimester. A heteroploid cell can arise due to endo reduplication ie. chromosomal replication without subsequent cell division and is mostly a cultural artifact.
Abnormal ultrasound findings are a common indication where rapid karyotyping is requested for management. If the fetus has abnormalities of classical syndromes, for example IUGR, choroid plexus cysts, renal or cardiac malformations suggestive of trisomy 18 on ultrasound scanning, and on placental biopsy the karyotype report is normal, this could be a false negative result. In this case, fetal tissue (amniotic fluid or fetal blood) should be done to confirm the karyotypic pattern.
At any time if a chromosomal rearrangement is detected in a prenatal diagnostic sample, parental karyotype should be done as soon as possible. For further pregnancy management in case of all fetal tissue samples which show an abnormal chromosomal pattern, parental karyotyping should be considered to detect the sporadic or familial origin of the same.
References
Purandarey, H. (2009). Essentials of Human Genetics. Second Edition. Jaypee Brothers Medical Publishers (P) Ltd.



|
|
|
|
صنع الذكريات والتفكير يدمر الدماغ.. دراسة تشرح السبب
|
|
|
|
|
|
|
الصين.. عودة كاسحتي الجليد إلى شنغهاي بعد انتهاء بعثة استكشافية إلى القطب الجنوبي
|
|
|
|
|
|
جامعة الكفيل تكرم الفائزين بأبحاث طلبة كلية الصيدلة وطب الأسنان
|
|
|
|
مشروع التكليف الشرعي بنسخته السادسة الورود الفاطمية... أضخم حفل لفتيات كربلاء
|
|
|
|
ضمن جناح جمعيّة العميد العلميّة والفكريّة المجمع العلمي يعرض إصداراته في معرض تونس الدولي للكتاب
|
|
|
|
جامعة الكفيل تعقد مؤتمرها الطلابي العلمي الرابع
|