


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-9-2020
Date: 11-10-2019
Date: 29-7-2018
|
Although direct alkylation of ammonia by alkyl halides leads to 1º-amines, alternative procedures are preferred in many cases. These methods require two steps, but they provide pure product, usually in good yield. The general strategy is to first form a carbon-nitrogen bond by reacting a nitrogen nucleophile with a carbon electrophile. The following table lists several general examples of this strategy in the rough order of decreasing nucleophilicity of the nitrogen reagent. In the second step, extraneous nitrogen substituents that may have facilitated this bonding are removed to give the amine product.
|
Nitrogen |
Carbon |
1st Reaction |
Initial Product |
2nd Reaction |
2nd Reaction |
Final Product |
|---|---|---|---|---|---|---|
| N3(–) | RCH2-X or R2CH-X |
SN2 | RCH2-N3 or R2CH-N3 |
LiAlH4 or 4 H2 & Pd |
Hydrogenolysis | RCH2-NH2 or R2CH-NH2 |
| C6H5SO2NH(–) | RCH2-X or R2CH-X |
SN2 | RCH2-NHSO2C6H5 or R2CH-NHSO2C6H5 |
Na in NH3 (liq) | Hydrogenolysis | RCH2-NH2 or R2CH-NH2 |
| CN(–) | RCH2-X or R2CH-X |
SN2 | RCH2-CN or R2CH-CN |
LiAlH4 | Reduction | RCH2-CH2NH2 or R2CH-CH2NH2 |
| NH3 | RCH=O or R2C=O |
Addition / Elimination |
RCH=NH or R2C=NH |
H2 & Ni or NaBH3CN |
Reduction | RCH2-NH2 or R2CH-NH2 |
| NH3 | RCOX | Addition / Elimination |
RCO-NH2 | LiAlH4 | Reduction | RCH2-NH2 |
| NH2CONH2 (urea) |
R3C(+) | SN1 | R3C-NHCONH2 | NaOH soln. | Hydrolysis | R3C-NH2 |
A specific example of each general class is provided in the diagram below. In the first two, an anionic nitrogen species undergoes an SN2 reaction with a modestly electrophilic alkyl halide reactant. For example #2 an acidic phthalimide derivative of ammonia has been substituted for the sulfonamide analog listed in the table. The principle is the same for the two cases, as will be noted later. Example #3 is similar in nature, but extends the carbon system by a methylene group (CH2). In all three of these methods 3º-alkyl halides cannot be used because the major reaction path is an E2 elimination.
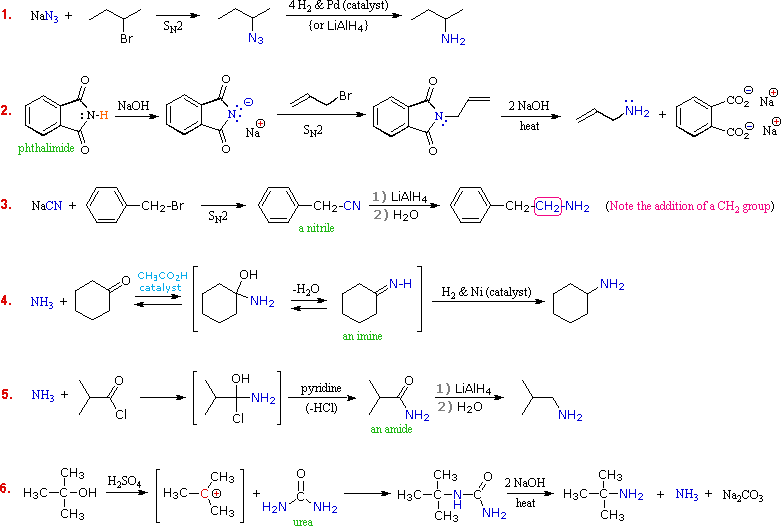
The methods illustrated by examples #4 and #5 proceed by attack of ammonia, or equivalent nitrogen nucleophiles, at the electrophilic carbon of a carbonyl group. A full discussion of carbonyl chemistry is presented later, but for present purposes it is sufficient to recognize that the C=O double bond is polarized so that the carbon atom is electrophilic. Nucleophile addition to aldehydes and ketones is often catalyzed by acids. Acid halides and anhydrides are even more electrophilic, and do not normally require catalysts to react with nucleophiles. The reaction of ammonia with aldehydes or ketones occurs by a reversible addition-elimination pathway to give imines (compounds having a C=N function). These intermediates are not usually isolated, but are reduced as they are formed (i.e. in situ). Acid chlorides react with ammonia to give amides, also by an addition-elimination path, and these are reduced to amines by LiAlH4.
The 6th example is a specialized procedure for bonding an amino group to a 3º-alkyl group (none of the previous methods accomplishes this). Since a carbocation is the electrophilic species, rather poorly nucleophilic nitrogen reactants can be used. Urea, the diamide of carbonic acid, fits this requirement nicely. The resulting 3º-alkyl-substituted urea is then hydrolyzed to give the amine. One important method of preparing 1º-amines, especially aryl amines, uses a reverse strategy. Here a strongly electrophilic nitrogen species (NO2(+)) bonds to a nucleophilic carbon compound. This nitration reaction gives a nitro group that can be reduced to a 1º-amine by any of several reduction procedures.
The Hofmann rearrangement of 1º-amides provides an additional synthesis of 1º-amines.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|