x

هدف البحث
بحث في العناوين
بحث في المحتوى
بحث في اسماء الكتب
بحث في اسماء المؤلفين

اختر القسم
موافق


النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الحيوية

مواضيع عامة في المضادات الحيوية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
trans-Splicing Reactions Use Small RNAs
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
16-5-2021
1219
trans-Splicing Reactions Use Small RNAs
KEY CONCEPTS
- Splicing reactions usually occur only in cis between splice sites on the same molecule of RNA.
- trans-splicing occurs in trypanosomes and worms where a short sequence (SL RNA) is spliced to the 5′ ends of many precursor mRNAs.
- SL RNAs have a structure resembling the Sm-binding site of U-snRNAs.
In mechanistic and evolutionary terms, splicing has been viewed as an intramolecular reaction, essentially amounting to a controlled deletion of the intron sequences at the level of RNA. In genetic terms, splicing is expected to occur only in cis. This means that only sequences on the same molecule of RNA should be spliced together.
The upper part of FIGURE 1 shows the usual situation. The introns can be removed from each RNA molecule, allowing the exons of that RNA molecule to be spliced together, but there is no intermolecular splicing of exons between different RNA molecules. Although we know that trans-splicing between pre-mRNA transcripts of the same gene does occur, it must be exceedingly rare, because if it were prevalent the exons of a gene would be able to complement one another genetically instead of belonging to a single complementation group.
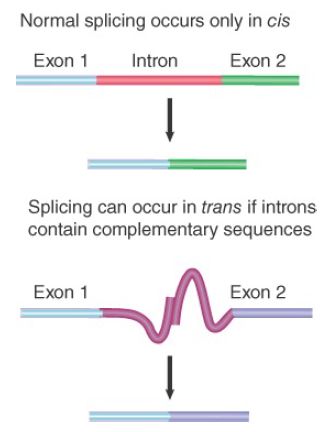
FIGURE 1. Splicing usually occurs only in cis between exons carried on the same physical RNA molecule, but trans-splicing can occur when special constructs that support base pairing between introns are made.
Some manipulations can generate trans-splicing. In the example illustrated in the lower part of Figure 1, complementary sequences were introduced into the introns of two RNAs. Base pairing between the complements should create an H-shaped molecule. This molecule could be spliced in cis, to connect exons that are covalently connected by an intron, or it could be spliced in trans, to connect exons of the juxtaposed RNA molecules. Both reactions occur in vitro.
Another situation in which trans-splicing is possible in vitro occurs when substrate RNAs are provided in the form of one containing a 5′ splice site and the other containing a 3′ splice site together with appropriate downstream sequences (which may be either the next 5′ splice site or a splicing enhancer). In effect, this mimics splicing by exon definition and shows that in vitro it is not necessary for the left and right splice sites to be on the same RNA molecule.
These results show that there is no mechanistic impediment to trans-splicing. They exclude models for splicing that require processive movement of a spliceosome along the RNA. It must be possible for a spliceosome to recognize the 5′ and 3′ splice sites of different RNAs when they are in close proximity.
Although trans-splicing is rare in multicellular eukaryotes, it occurs as the primary mechanism to process precursor RNA into mature, translatable mRNAs in some organisms, such as trypanosomes and nematodes. In trypanosomes, all genes are expressed as polycistronic transcripts, like those in bacteria. However, the transcribed RNA cannot be translated without a 37-nucleotide leader brought in by trans-splicing to convert a polycistronic RNA into individual monocistronic mRNAs for translation. The leader sequence is not encoded upstream of the individual transcription units, though. Instead, it is transcribed into an independent RNA,
carrying additional sequences at its 3′ end, from a repetitive unit located elsewhere in the genome. FIGURE 2 shows that this RNA carries the leader sequence followed by a 5′ splice-site sequence. The sequences encoding the mRNAs carry a 3′ splice site just preceding the sequence found in the mature mRNA.
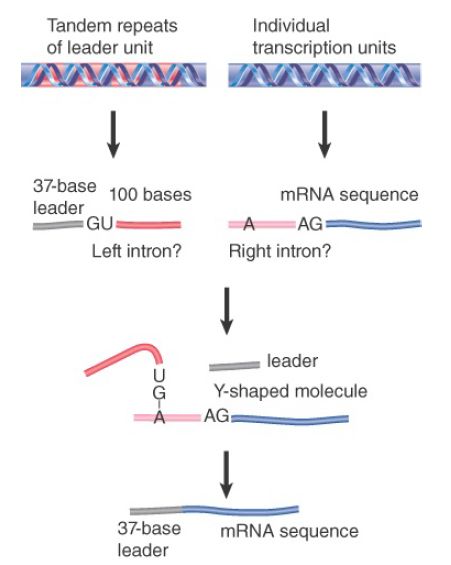
FIGURE 2. The SL RNA provides an exon that is connected to the first exon of an mRNA by trans-splicing. The reaction involves the same interactions as nuclear cis-splicing but generates a Yshaped RNA instead of a lariat.
When the leader and the mRNA are connected by a trans-splicing reaction, the 3′ region of the leader RNA and the 5′ region of the mRNA in effect comprise the 5′ and 3′ halves of an intron. When splicing occurs, a 2′–5′ link forms by the usual reaction between the GU of the 5′ intron and the branch sequence near the AG of the 3′ intron. The two parts of the intron are covalently linked, but generate a Y-shaped molecule instead of a lariat.
The RNA that donates the 5′ exon for trans-splicing is called the spliced leader RNA (SL RNA). The SL RNAs, which are 100 nucleotides in length, can fold into a common secondary structure that has three stem-loops and a single-stranded region that resembles the Sm-binding site. The SL RNAs therefore exist as snRNPs that count as members of the Sm snRNP class. During the trans-splicing reaction, SL RNA becomes part of the spliced product replacing the original cap and leader (called an outron), as illustrated in the upper panel of FIGURE 3. Like other snRNPs involved in splicing (except U6), SL RNA carries a trimethylated cap, which is recognized by the variant cap-binding factor eIF4E to facilitate translation.
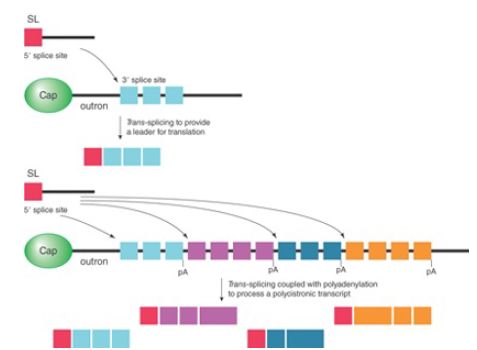
FIGURE 3. The SL RNA adds a leader to facilitate translation. Coupled with the cleavage and polyadenylation reactions, the addition of the SL RNA is also used to convert polycistronic transcripts to monocistronic units.
In Caenorhabditis elegans, about 70% of genes are processed by the trans-splicing mechanism, which can be further divided into two classes of genes. One class produces monocistronic transcripts that are processed by both cis- and trans-splicing. In these cases, cis-splicing is used to remove internal intronic sequences, and then trans-splicing is employed to provide the 22-nucleotide leader sequence derived from the SL RNA for translation. The other class is polycistronic. In these cases, trans-splicing is used to convert the polycistronic transcripts into monocistronic transcripts in addition to providing the SL leader sequence for their translation, as illustrated in the bottom panel of Figure 3.
C. elegans has two types of SL RNA. SL1 RNA (the first to be discovered) is only used to remove the 5′ ends of pre-mRNAs transcribed from monocistronic genes. How does the SL RNA find the 3′ splice site to initiate trans-splicing, and in doing so, how does trans-splicing avoid competition or interference with cis-splicing? The ability to target a functional 3′ splice site is provided by the proteins as part of the SL snRNP. For example, purified SL snRNP from Ascaris, a parasitic nematode, contains two specific proteins, one of which (SL-30kD) can directly interact with the BPB protein at the 3′ splice site. The SL1 RNA is only trans-spliced to the first 5′ untranslated region, and does not interfere with downstream cissplicing events. This is because only the 5′ untranslated region contains a functional 3′ splice site, but it does not have the upstream 5′ splice site to pair with the downstream 3′ splice site.
The SL2 RNA is used in most cases to process polycistronic transcripts that are separated by a 100-nucleotide spacer sequence between the two adjacent gene units. In a small fraction of genes where the two adjacent gene units are linked without any spacer sequences, the SL1 RNA is used to break them up. During processing of these polycistronic transcripts by either of the SL snRNAs, the trans-splicing reaction is tightly coupled with the cleavage and polyadenylation reactions at the end of each gene unit. Such coupling appears to be facilitated by direct protein–protein interactions between the SL2 snRNP and the cleavage stimulatory factor CstF that binds to the U-rich sequence downstream of the AAUAAA signal (see the next section, The 3′ Ends of mRNAs Are Generated by Cleavage and Polyadenylation). These mechanisms allow related genes to be coregulated at the level of transcription (because they are transcribed as polycistronic transcripts) and individually regulated after transcription (because individual gene units are separated as a result of RNA processing).